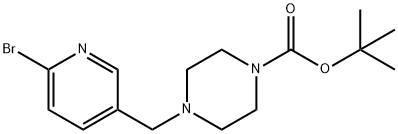
tert-Butyl 4-((6-bromopyridin-3-yl)methyl)piperazine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-((6-bromopyridin-3-yl)methyl)piperazine-1-carboxylate
- CAS Number:1160923-86-3
- Molecular formula:C15H22BrN3O2
- Molecular Weight:356.26
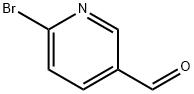
149806-06-4
436 suppliers
$5.00/1g
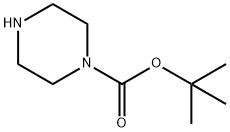
57260-71-6
749 suppliers
$5.00/5g

1160923-86-3
19 suppliers
inquiry
Yield:1160923-86-3 78%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in dichloromethane at 15 - 25; for 24 h;
Steps:
t-Butyl 4-((6-bromopyridin-3-yl)methyl)piperazine-l-carboxylate (iii)
A solution of 5-bromopyridine-2-carbaldehyde (i; 71.4 g, 384 mmol, 1 equiv), 1-Boc- piperzine (ii; 72.8 g, 391 mmol, 1.02 equiv), dichloromethane (1.2 L), and acetic acid (2.4 mL) was cooled to 15 °C and sodium triacetoxyborohydride (164.9 g, 777.8 mmol, 2.03 equiv) was added portionwise to the mixture at 25 °C. The resulting solution was stirred for 24 h at room temperature. An additional portion of sodium triacetoxyborohydride (13 g, 61.3 mmol, 0.16 equiv) was added portionwise at 15 °C. The reaction was then quenched with aq 2N NaOH (250 mL) and the resulting solution was stirred for another 0.5 h. The organic layer was washed twice with water, dried and concentrated. The residue was recrystallized with 4:1 petroleum ether/ethyl acetate to yield 107.7 g (78% yield) of iii as a light yellow solid.
References:
WO2019/148161,2019,A1 Location in patent:Page/Page column 22; 23