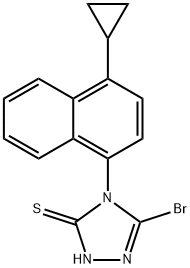
3-bromo-4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione synthesis
- Product Name:3-bromo-4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione
- CAS Number:1158970-76-3
- Molecular formula:C15H12BrN3S
- Molecular Weight:346.24
![Propanoic acid, 3-[[5-bromo-4-(4-cyclopropyl-1-naphthalenyl)-4H-1,2,4-triazol-3-yl]thio]-, ethyl ester](/CAS/20200611/GIF/1158970-26-3.gif)
1158970-26-3
0 suppliers
inquiry

1158970-76-3
17 suppliers
inquiry
Yield:1158970-76-3 78%
Reaction Conditions:
Stage #1: ethyl 3-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)propanoatewith lithium hydroxide;water in tetrahydrofuran;methanol at 20; for 2 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water;
Steps:
3.C
[00330] Step B: Lithium hydroxide solution (IM aqueous, 0.88mL, 0.488 mmol) was added to a solution of 4 ethyl 2-(4-(4,7-dimethylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetate (0.15 g, 0.44 mmol) in THF/ethanol/water (1 : 1 :1, 7mL) and the mixture stirred for 2h at room temperature. The crude reaction mixture was then concentrated, acidified with HCl (IM, 3mL) and extracted with ethyl acetate (3x5mL). The combined organics extracts were concentrated to afford 2-(4-(4,7- dimethylnaphthalen-1-yl)-4H- 1,2,4-triazol-3-ylthio)acetic acid as an off-white solid (0.129 g, 94%),
References:
WO2009/70740,2009,A2 Location in patent:Page/Page column 76-77
127971-24-8
11 suppliers
inquiry

1158970-76-3
17 suppliers
inquiry
1533519-84-4
184 suppliers
$12.00/250mg

1158970-76-3
17 suppliers
inquiry