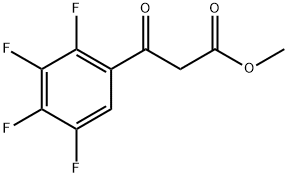
Benzenepropanoic acid, 2,3,4,5-tetrafluoro-β-oxo-, methyl ester synthesis
- Product Name:Benzenepropanoic acid, 2,3,4,5-tetrafluoro-β-oxo-, methyl ester
- CAS Number:111630-16-1
- Molecular formula:C10H6F4O3
- Molecular Weight:250.15
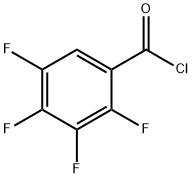
94695-48-4
259 suppliers
$10.00/5G
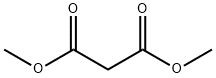
108-59-8
602 suppliers
$5.00/25g
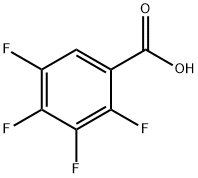
1201-31-6
403 suppliers
$10.00/5g

111630-16-1
1 suppliers
inquiry
Yield:111630-16-1 3.25 g (45-50%)
Reaction Conditions:
with ammonium hydroxide;Hg;sodium methylate in N-methyl-acetamide;water;toluene;
Steps:
1 EXAMPLE 1
EXAMPLE 1 A 500 ml, three-necked flask was set up in a heating mantle and equipped with a mechanical stirrer, an equilibrating type addition funnel, a thermometer, an inert gas (argon or nitrogen) inlet, and a distillation head set for downward distillation. The flask was charged with 100 ml of toluene, 2 ml of dimethylformamide, and 2.84 g (0.0525 mol) of anhydrous sodium methoxide. The slurry was heated with stirring to 110° C., and dimethylmalonate (7.1 g, 0.0538 mol) was added dropwise from the addition funnel over 15 to 20 minutes. A total of 20 ml of distillate was collected at a vapor temperature of 50°-105° C. A fluid slurry was obtained. After five minutes at 105° C. (vapor), the mixture was cooled to 18°-20° C., and 5.3 g (0.025 mol) of 2,3,4,5-tetrafluorobenzoyl chloride was added dropwise with good stirring. The mixture was stirred for three hours at ambient temperature. Water (250 ml) was added with stirring and the aqueous layer was removed and acidified to a pH of 2-3 with 2N HCl. The organic product was extracted with two 50 ml portions of methylene chloride. The extract was dried over magnesium sulfate and evaporated to dryness. The residue was distilled under reduced pressure (0.2-0.3 mm of Hg) to remove dimethyl malonate (pot temperature 85°-105° C., vapor 35°-39° C.). A gas chromatographic analysis of the residue indicated that it contained 94-97% of the desired product. The oily residue was mixed with 140 ml of water and refluxed for 1.5 hours. The mixture was cooled to room temperature and extracted with two 50 ml portions of methylene chloride. The organic layer was washed with 20 ml portions of 2.4N ammonium hydroxide solution till the aqueous extracts showed a pH of 9. The methylene chloride layer was dried over magnesium sulfate, concentrated to dryness, and triturated with cold n-hexane. The product was collected and dried under vacuum to yield 3.25 g (45-50%) of methyl 2,3,4,5-tetrafluorobenzoylacetate, mp 68°-72° C., which was 98% pure by gas chromatographic analysis. A pure sample was prepared by recrystallization from n-hexane, mp 71°-74° C. The aqueous basic layer was acidified to pH 2 with 2N HCl. The by-product 2,3,4,5-tetrafluorobenzoic acid was isolated by extraction with methylene chloride to give 1.3 g (26%) as a white crystalline solid, mp 81°-83° C. This was converted to tetrafluorobenzoyl chloride and recycled in the synthesis, Step 2.
References:
US4689423,1987,A