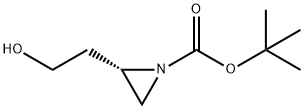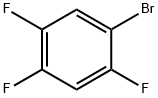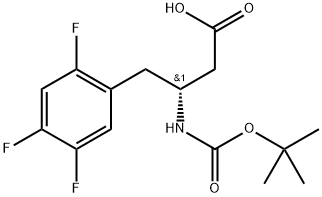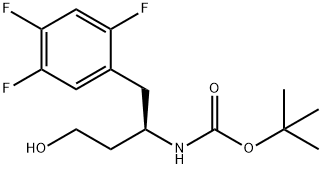
Carbamic acid, N-[(1R)-3-hydroxy-1-[(2,4,5-trifluorophenyl)methyl]propyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-[(1R)-3-hydroxy-1-[(2,4,5-trifluorophenyl)methyl]propyl]-, 1,1-dimethylethyl ester
- CAS Number:1048703-14-5
- Molecular formula:C15H20F3NO3
- Molecular Weight:319.32
Yield:1048703-14-5 77%
Reaction Conditions:
Stage #1: 1-bromo-2,4,5-trifluorobenzenewith isopropylmagnesium bromide in tetrahydrofuran at -20;Inert atmosphere;
Stage #2: with copper(I) bromide dimethylsulfide complex in tetrahydrofuran at -5; for 0.25 h;Inert atmosphere;
Stage #3: (2S)-2-(2-hydroxy-ethyl)-aziridine-1-carboxylic acid tert-butyl esterFurther stages;
Steps:
40 Example 40
Into a 100 mL three-necked flask were added 4.62 g 1-bromo-2,4,5-trifluorobenzene (0.022 mol) and anhydrous tetrahydrofuran (50 mL). The resulting mixture was cooled to -20° C. The solution of isopropylmagnesium bromide (22 mmol) in tetrahydrofuran (22 ml, 1M THF) was slowly added dropwise under nitrogen. After the addition was complete, the reactants were maintained at -20° C. for later use. Cuprous bromide-dimethyl sulfide (0.41 g, 0.002 mol) was suspended in 5 ml anhydrous tetrahydrofuran. The resulting mixture was cooled to -5° C. The Grignard reagent as described above was slowly added dropwise under nitrogen. After 15 min, a solution of the aziridine compound as shown in the above reaction formula (2.81 g, 0.015 mol) in 30 mL tetrahydrofuran was slowly added dropwise. After additional 5 min, 50 mL saturated solution of ammonia chloride was added to quench the reaction. Into this obtained solution was added 50 mL ethyl acetate. The separated water layer was extracted with another 50 mL ethyl acetate. The obtained organic layers were collected together and further washed with saturated solution of sodium chloride and then dried over anhydrous sodium sulfate, followed by filtration and concentration to obtain a crude product, which was further treated by column chromatography to obtain a compound (3.69 g, 0.0115 mol, yield 77%). 1H NMR (500 MHz, CDCl3) δ 7.05 (d, J=8.3 Hz, 1H), 6.91 (d, J=6.6 Hz, 1H), 4.56 (d, J=9.0 Hz, 1H), 4.133.96 (m, 1H), 3.68 (d, J=6.0 Hz, 2H), 2.852.65 (m, 2H), 1.86 (dd, J=12.4, 7.7 Hz, 1H), 1.68 (s, 1H), 1.42 (s, 9H). Ms (M++1): 320.
References:
US2013/281695,2013,A1 Location in patent:Paragraph 0221-0224
![Carbamic acid, N-[(1R)-3-(methylthio)-1-[(2,4,5-trifluorophenyl)methyl]propyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1380243-28-6.gif)
1380243-28-6
0 suppliers
inquiry
![Carbamic acid, N-[(1R)-3-hydroxy-1-[(2,4,5-trifluorophenyl)methyl]propyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/1048703-14-5.gif)
1048703-14-5
0 suppliers
inquiry
![Benzenebutanoicacid,b-[[(1,1-diMethylethoxy)carbonyl]aMino]-2,4,5-trifluoro-,Methylester,(bR)-](/CAS/20150408/GIF/881995-73-9.gif)
881995-73-9
41 suppliers
inquiry
![Carbamic acid, N-[(1R)-3-hydroxy-1-[(2,4,5-trifluorophenyl)methyl]propyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/1048703-14-5.gif)
1048703-14-5
0 suppliers
inquiry
![Carbamic acid, N-[(1S)-3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-1-(hydroxymethyl)propyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/191655-46-6.gif)
191655-46-6
0 suppliers
inquiry
![Carbamic acid, N-[(1R)-3-hydroxy-1-[(2,4,5-trifluorophenyl)methyl]propyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/1048703-14-5.gif)
1048703-14-5
0 suppliers
inquiry