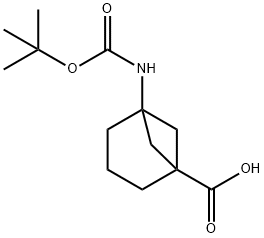
5-[(TERT-BUTOXYCARBONYL)AMINO]BICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID synthesis
- Product Name:5-[(TERT-BUTOXYCARBONYL)AMINO]BICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID
- CAS Number:1035325-28-0
- Molecular formula:C13H21NO4
- Molecular Weight:255.31
![methyl 5-{[(tert-butoxy)carbonyl]amino}bicyclo[3.1.1]heptane-1-carboxylate](/CAS/20200401/GIF/1035325-26-8.gif)
1035325-26-8
7 suppliers
inquiry
![5-[(TERT-BUTOXYCARBONYL)AMINO]BICYCLO[3.1.1]HEPTANE-1-CARBOXYLIC ACID](/CAS/20200401/GIF/1035325-28-0.gif)
1035325-28-0
11 suppliers
inquiry
Yield:1035325-28-0 85%
Reaction Conditions:
Stage #1: 5-tert-butoxycarbonylamino-bicyclo[3.1.1]heptane-1-carboxylic acid methyl esterwith lithium hydroxide;water in methanol at 0; for 48 h;
Stage #2: with citric acid in water;pentane; pH=5;
Steps:
51
5-tert-Butoxycarbonylamino-bicyclo[3.1.1]heptane-1-carboxylic acid. Solid LiOH (2.18 g, 51.98 mmol) was added portion wise to a solution of 5-tert- Butoxycarbonylamino-bicyclo[3.1.1]heptane-1-carboxylic acid methyl ester (3.5 g, 12.09 mmol) in 80 % aqueous MeOH (14 ml) at O0C. The reaction mixture was stirred for 48h, evaporated to remove the alcohol. The crude residue was diluted with water, washed with pentane and then acidified with 10 % citric acid (pH-5). It was extracted with dichloromethane, dried over sodium sulfate and evaporated to afford the desired product (2.8 g, 85 %) as an off white solid.1H NMR (400 MHz, DMSO-d6): δ 12.2 (br s, 1 H), 6.97 (br s, 1 H), 2.29 (br m, 2H), 1.78 -1.75 (m, 8H), 1.37 (s, 9H).
References:
WO2008/75196,2008,A1 Location in patent:Page/Page column 113