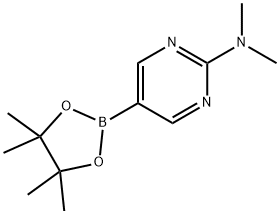
2-Dimethylamino-pyrimidine-5-boronic acid pinacol ester synthesis
- Product Name:2-Dimethylamino-pyrimidine-5-boronic acid pinacol ester
- CAS Number:1032759-30-0
- Molecular formula:C12H20BN3O2
- Molecular Weight:249.12
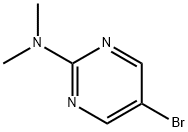
38696-21-8
118 suppliers
$16.00/1g
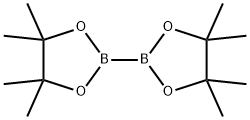
73183-34-3
583 suppliers
$6.00/5g

1032759-30-0
69 suppliers
$23.00/100mg
Yield:1032759-30-0 60%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane;1,2-dimethoxyethane at 150; for 0.25 h;Inert atmosphere;Microwave irradiation;
Steps:
N,N-dimeth l-5-(4,4,5,5-tetrameth l-1,3,2-dioxaborolan-2- l)p rimidin-2-amine (10a)
A mixture of 5-bromo-N,N-dimethylpyrimidin-2-amine (0.966 g, 4.78 mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(1,3,2-dioxaborolane) (1.46 g, 5.74 mmol), PdCl2dppf(DCM) (0.114 g, 0.14 mmol) and KOAc (1.407 g, 14.34 mmol) were placed under Ar in a 20 mL microwave flask. Anhydrous 1,2-dimethoxyethane (16 mL) was added and the flask was irradiated at 150 oC for 15 minutes. The reaction was filtered through celite, concentrated and slurried in EtOAc. The reaction was filtered through celite again and the organics were concentrated and purified by column chromatography 0 to 30 % EtOAC in Hexanes. The material was isolated as a light teal solid (0.718 g, 60 % yield). 1H NMR (400 MHz, CDCl3) δ 8.59 (s, 2H), 3.21 (s, 6H), 1.31 (s, 12H).13C NMR (100 MHz, CDCl3) δ 164.1, 83.7, 77.4, 37.2, 25.2, 24.9. HRMS (EI+) m/z calculated for C12H12BN3O2 [M+H]+: 250.1721, found: 250.17189.
References:
WO2017/197151,2017,A1 Location in patent:Page/Page column 39-40