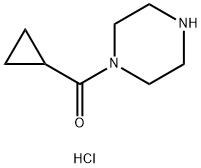
Piperazine, 1-(cyclopropylcarbonyl)-, Monohydrochloride synthesis
- Product Name:Piperazine, 1-(cyclopropylcarbonyl)-, Monohydrochloride
- CAS Number:1021298-67-8
- Molecular formula:C8H15ClN2O
- Molecular Weight:190.67
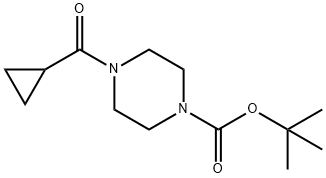
414910-15-9
100 suppliers
$12.00/1mg

1021298-67-8
234 suppliers
$17.00/250mg
Yield: 100%
Reaction Conditions:
with hydrogenchloride in methanol at 0 - 20;
Steps:
17B
To a stirred mixture of compound tert-butyl 4-(cyclopropanecarbonyl)piperazine-1-carboxylate (3.7 g, 14.5 mmol) in methanol (15 mL) was added hydrochloride/methanol (15 mL, 3M)) at 0° C. After the addition, the mixture was allowed to stir at room temperature overnight. The mixture was concentrated to give cyclopropyl(piperazin-1-yl)methanone hydrochloride (2.74 g, yield 100%) as an off-white solid. 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 0.71-0.76 (m, 4H), 1.96-2.03 (m, 1H), 3.04-3.16 (m, 4H), 3.69-4.08 (m, 4H), 9.58 (s, 2H); LC-MS (ESI) m/z: 155(M+1)+
References:
LEAD THERAPEUTICS, INC. US2010/35883, 2010, A1 Location in patent:Page/Page column 56
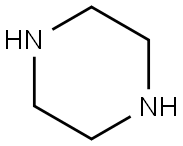
110-85-0
564 suppliers
$5.00/5 g
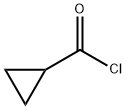
4023-34-1
361 suppliers
$12.00/5g

1021298-67-8
234 suppliers
$17.00/250mg