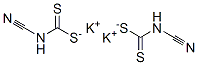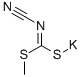
CYANIMIDODITHIOCARBONIC ACID MONOMETHYL ESTER MONOPOTASSIUM SALT synthesis
- Product Name:CYANIMIDODITHIOCARBONIC ACID MONOMETHYL ESTER MONOPOTASSIUM SALT
- CAS Number:10191-61-4
- Molecular formula:C3H3KN2S2
- Molecular Weight:170.3
Yield: 74%
Reaction Conditions:
in water;acetone at 0 - 20; for 2 h;
Steps:
115a
PREPARATION 115a3-Bromo-5-methanesulfonyl-[1 ,2,4]thiadiazoleA mixture of cyanamide (10.6 g, 0.25 mol) and CS2 (21.0 g, 0.276 mol) was stirred at room temperature for 30 minutes. Then KOH (28.1 g, 0.5 mol) in 95% EtOH (90 mL) was added dropwise to the mixture at 0°C. After addition, the resulting mixture was stirred at room temperature overnight. The reaction mixture filtered and the wet cake washed with EtOH (10 mL) to give potassium cyanocarbonimidodithioate (20 g, 41.2%) as a white solid.To a solution of potassium cyanocarbonimidodithioate (10 g, 51.5 mmol) in acetone (40 mL) and water (45 mL) was added dropwise Mel (7.31 g, 51.5 mmol) at 0°C. After addition, the resulting mixture was stirred at room temperature for 2 hours. TLC(Petroleum ethenEtOAc 0:1 ) indicated the reaction was completed. The reaction mixture was concentrated in vacuum and to the was added acetone (100 mL) and then stirred at room temperature for 0.5 hour. The mixture was filtered and the filtrate wasconcentrated in vacuo to give crude potassium methyl cyanocarbonimidodithioate, which was crystallized from MTBE (20 mL) to give potassium methylcyanocarbonimidodithioate (6.5 g,74%) as a white solid.To a solution of potassium methyl cyanocarbonimidodithioate (5 g, 29.4 mmol) in dichloromethane (100 mL) was added dropwise Br2 (5.2 g, 32.9 mmol) at 0 °C. After addition, the resulting mixture was stirred at room temperature overnight. TLC(Petroleum ether: EtOAc 0:1 ) indicated the reaction was completed. The reaction mixture was added excess Na2S03 and water (50 mL) to decompose the excess Br2. The mixture was separated and the separated organic layer was washed with brine (50 mL x 3), dried over Na2S0 and concentrated in vacuo to yield 3-bromo-5- methylsulfanyl-[1 ,2,4]thiadiazole (1.2 g, 19.2%) as an off-white solid.To a solution of 3-bromo-5-methylsulfanyl-[1 ,2,4]thiadiazole (0.8 g, 3.8 mmol) in dichloromethane (20 mL) was added dropwise mCPBA (1.97 g, 11.4 mmol) in DCM (10 mL) at 0°C. After addition, the resulting mixture was stirred at room temperature overnight. TLC (Petroleum ethenEtOAc 10:1 ) indicated the reaction was completed. The reaction mixture was added excess sodium sulfite and water (50 mL) to decompose the excess mCPBA. The mixture was separated and the separated organic layer was washed with brine (50 mL χ 3), dried over sodium sulfate and concentrated in vacuo to give residue, which was purified by a silica gel column to yield the title compound (0.58 g, 63.2%) as a white solid.
References:
PFIZER INC.;BUNNAGE, Mark, Edward;COOK, Andrew, Simon;CUI, Jingrong, Jean;DACK, Kevin, Neil;DEAL, Judith, Gail;GU, Danlin;HE, Mingying;JOHNSON, Patrick, Stephen;JOHNSON, Ted, William;LE, Phuong, Thi, Quy;PALMER, Cynthia, Louise;SHEN, Hong WO2011/138751, 2011, A2 Location in patent:Page/Page column 120-121