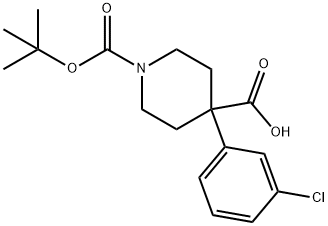
1-BOC-4-(3-CHLOROPHENYL)-4-PIPERIDINEDICARBOXYLIC ACID synthesis
- Product Name:1-BOC-4-(3-CHLOROPHENYL)-4-PIPERIDINEDICARBOXYLIC ACID
- CAS Number:1001124-90-8
- Molecular formula:C17H22ClNO4
- Molecular Weight:339.81

24424-99-5
833 suppliers
$13.50/25G

1001124-90-8
13 suppliers
$365.00/1G
Yield:1001124-90-8 75.56%
Reaction Conditions:
Stage #1: tert-butyl 4-(3-chlorophenyl)-4-cyanopiperidine-1-carboxylatewith hydrogenchloride;waterHeating / reflux;
Stage #2: di-tert-butyl dicarbonatewith sodium hydroxide in 1,4-dioxane;water at 20; for 14 h;
Steps:
26.2
Step 2: tert-Butyl 4-(3-chlorophenyl)-4-cyanopiperidine-l-carboxylate (5.91 g,18.42 mmol) was dissolved in concentrated HCl (153.5 ml, 1842 mmol). The reaction mixture stirred at reflux over a weekend. The reaction mixture was cooled to room temperature and washed with ether. The aqueous portion was concentrated on a rotary evaporator, and the solids were dried on a high vacuum line. The solids were dissolved in H2O (35 mL), 10% NaOH (29.47 g, 73.69 mmol), and dioxane (30 mL). Solid Boc2O (4.222 g, 19.34 mmol) was added, and reaction mixture stirred at room temperature overnight (14 hours). The reaction mixture was diluted with H2O and washed with ether. The aqueous portion was acidified with solid KHSO4, then extracted with DCM. The combined extracts were dried (Na2SO4), filtered, and concentrated to give l-(tert- butoxycarbonyl)-4-(3-chlorophenyl)piperidine-4-carboxylic acid (4.73 g, 75.56% yield) as a white powder. HPLC >98%. LC/MS (APCI-) m/z 338 [M-H]'.
References:
WO2008/6039,2008,A1 Location in patent:Page/Page column 92