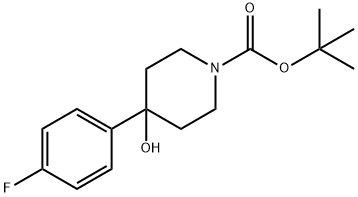
1-N-BOC-4-(4-FLUOROPHENYL)-4-HYDROXYPIPERIDINE synthesis
- Product Name:1-N-BOC-4-(4-FLUOROPHENYL)-4-HYDROXYPIPERIDINE
- CAS Number:553631-05-3
- Molecular formula:C16H22FNO3
- Molecular Weight:295.35
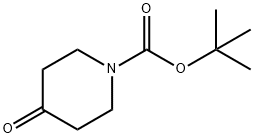
79099-07-3
543 suppliers
$5.00/5g
352-13-6
195 suppliers
$40.00/50mL

553631-05-3
25 suppliers
$146.66/1g
Yield:553631-05-3 75.6%
Reaction Conditions:
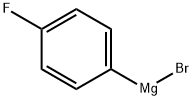
Stage #1: N-tert-butyloxycarbonylpiperidin-4-one;4-flourophenylmagnesium bromide in tetrahydrofuran at 0 - 20; for 16 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran at 20;Saturated solution;
Steps:
1
Synthesis of the Amine Unit AM-09: 4-(4-Fluorophenyl)-4-(2-pyrrolidin-1-yl-ethoxy)-piperidine-1-carboxylic acid tert-butyl ester (AM-09) Stage-1: A solution of N-Boc-piperidone (10 g, 50.188 mmol) in THF (100 ml) was added to a THF solution of 4-fluorophenylmagnesium bromide (100.376 mmol, 0.5 M) at 0° C. When the addition was complete, the reaction mixture was stirred at room temperature for 16 h (TLC control). The reaction was quenched with sat. NH4Cl solution, the reaction mixture was diluted with ethyl acetate and the organic phase was washed successively with H2O and sat. NaCl solution. The organic phase was dried over Na2SO4 and finally concentrated in vacuo to obtain the crude product, which was purified by column chromatography (2% methanol in methylene chloride). Yield: 75.6%
References:
US2009/264400,2009,A1 Location in patent:Page/Page column 65

79099-07-3
543 suppliers
$5.00/5g
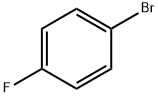
460-00-4
620 suppliers
$6.00/10g

553631-05-3
25 suppliers
$146.66/1g

79099-07-3
543 suppliers
$5.00/5g
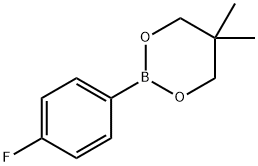
225916-39-2
49 suppliers
$75.00/500mg

553631-05-3
25 suppliers
$146.66/1g
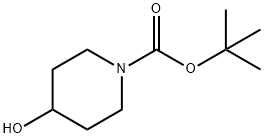
109384-19-2
474 suppliers
$5.00/1g

225916-39-2
49 suppliers
$75.00/500mg

553631-05-3
25 suppliers
$146.66/1g
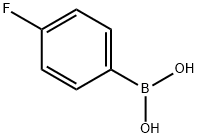
1765-93-1
557 suppliers
$9.00/1g

553631-05-3
25 suppliers
$146.66/1g