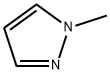
1-Methylpyrazole synthesis
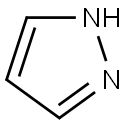
- Product Name:1-Methylpyrazole
- CAS Number:930-36-9
- Molecular formula:C4H6N2
- Molecular Weight:82.1
Yield:930-36-9 14%
Reaction Conditions:
at 115 - 140; for 8 - 9 h;
Steps:
4; 6 EXAMPLE 4; Synthesis of N-Methylpyrazole
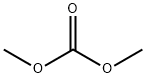
The apparatus used is the same as that described in Example 1. 20.64 g of pyrazole, i.e. 0.3 mol, and 4.5 g of dimethyl carbonate, i.e. 0.05 mol, were introduced into the reactor. The medium was then heated to 140° C. and this temperature was maintained throughout the reaction. The dimethyl carbonate was introduced into the reactor with a flow rate of 60 mmol/h for 8 hours and the methanol produced was distilled off as it was formed. The reaction medium was then allowed to cool to ambient temperature to obtain 17.24 g of N-methyyl pyrazole i.e. 0.21 mol, which corresponded to a yield of 70%.EXAMPLE 6 Synthesis of N-Methylpyrazole Without Withdrawal of the Methanol [0028] The apparatus used is the same as that described in Example 1. 20.64 g of pyrazole, i.e. 0.3 mol, and 4.5 g of dimethyl carbonate, i,e. 0.05 mol, were introduced into the reactor. The medium was then heated to 140° C. and then the dimethyl carbonate was introduced into the reactor with a flow rate of 60 mmol/h (i.e. 5.4 g/h) for 8 hours. The methanol formed was not withdrawn. [0029] It was found that the temperature of the reaction medium remained stable at 140° C. for 1 hour and then gradually decreased to 115° C. at the end of the reaction. The reactor medium was then allowed to cool to ambient temperature to obtain only 3.53 g of N-methylpyrazole, i.e. 0.042 mol, which corresponded to a yield of 14%.
References:
Borredon, Elisabeth;Chabaud, Berhard;Gaset, Antoine;Thiebaud-Roux, Sophie;Ouk, Samedy US2004/24205, 2004, A1 Location in patent:Page 2; 3
39806-90-1
227 suppliers
$9.00/5g

930-36-9
331 suppliers
$5.00/1g