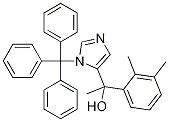
1’-Hydroxy N-Trityl Medetomidine synthesis
- Product Name:1’-Hydroxy N-Trityl Medetomidine
- CAS Number:176721-03-2
- Molecular formula:C32H30N2O
- Molecular Weight:458.59
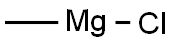
676-58-4
295 suppliers
$15.00/25ml
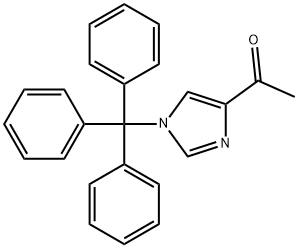
![(2,3-Dimethylphenyl)[1-(trityl)-1H-imidazol-4-yl]methanone](/CAS2/GIF/176721-02-1.gif)
176721-02-1
77 suppliers
$110.09/10mg

176721-03-2
51 suppliers
$105.00/10mg
Yield:176721-03-2 97.5%
Reaction Conditions:
in tetrahydrofuran at 0 - 25; for 3.5 h;
Steps:
3.c
Example 3 (step c); Preparation of 1-(2,3-dimethylphenyl)-1-(3-trityl-3H-imidazol-4-yl)ethanol (2,3-Dimethylphenyl)-(3-trityl-3H-imidazol-4-yl)methanone (216 g, 0.488 mol) was added to stirred tetrahydrofuran (3000 mL) in a 6-liter glass reactor fitted with a mechanical stirrer, a thermometer, a dropping funnel and a tube for argon introduction into the reaction mixture. A methylmagnesium chloride solution in tetrahydrofuran (190 mL, 0.584 mol) was added dropwise to the reaction mixture at 0°C under an argon atmosphere. The reaction mixture was maintained at 0°C. After addition of a methylmagnesium chloride solution the reaction mixture was warmed to 25°C over 3,5 hours, at which point 10 % aqueous ammonium chloride solution (90 mL) was added to the reaction mixture. The organic layer was separated and washed with saturated sodium chloride solution (700 mL). The organic layer was concentrated in vacuo to 20% of the original volume; the residue was cooled and allowed to crystallize at 0 to 5°C for 1 hour. The precipitate was separated by filtration and washed with cold (0 to 5°C) tetrahydrofuran (360 mL). The obtained intermediate 1-(2,3-dimethylphenyl)-1-(3-trityl-3H-imidazol-4-yl)ethanol was dried at a reduced pressure at 40-50°C. The yield was 214.2g (97.5 %) of white crystalline 1-(2,3-dimethylphenyl)-1-(3-trityl-3H-imidazol-4-yl)ethanol, having a melting temperature of 226.5°C to 228.5°C.
References:
EP1918282,2008,A1 Location in patent:Page/Page column 6
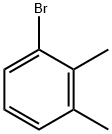
576-23-8
276 suppliers
$16.00/25g
116795-55-2
32 suppliers
inquiry

176721-03-2
51 suppliers
$105.00/10mg
![(2,3-Dimethylphenyl)[1-(trityl)-1H-imidazol-4-yl]methanone](/CAS2/GIF/176721-02-1.gif)
176721-02-1
77 suppliers
$110.09/10mg

176721-03-2
51 suppliers
$105.00/10mg
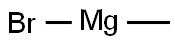
75-16-1
278 suppliers
$12.00/10ml
![(2,3-Dimethylphenyl)[1-(trityl)-1H-imidazol-4-yl]methanone](/CAS2/GIF/176721-02-1.gif)
176721-02-1
77 suppliers
$110.09/10mg

176721-03-2
51 suppliers
$105.00/10mg

576-23-8
276 suppliers
$16.00/25g

176721-03-2
51 suppliers
$105.00/10mg