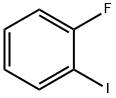
1-Fluoro-2-iodobenzene synthesis
- Product Name:1-Fluoro-2-iodobenzene
- CAS Number:348-52-7
- Molecular formula:C6H4FI
- Molecular Weight:222
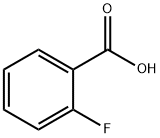
445-29-4
419 suppliers
$5.00/10g

348-52-7
292 suppliers
$6.00/5g
Yield:348-52-7 64%
Reaction Conditions:
with N-iodo-succinimide;[4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate;iodine;caesium carbonate in 1,2-dichloro-ethane at 50; for 24 h;Inert atmosphere;Irradiation;Sealed tube;
Steps:
General procedure A.
General procedure: To a 15 mL test tube with septum Cs2CO3 (0.6 mmol, 195 mg), aromaticcarboxylic acid (1) (0.3 mmol), [Ir(dF(CF3)ppy)2dtbbpy]PF6 (D) (6 μmmol, 6.7 mg), Niodosuccinimide(NIS) (0.9 mmol, 202.5 mg) and I2 (15 μmol, 5 mol%) were added. The tube was evacuated and backfilled with argon for three times, and then 3 mL of dry 1,2-dichloroethane(DCE) was added through a syringer under argon. The tube was sealed with Parafilm M andplaced in an oil bath with a contact thermometer, and the reaction was carried out at 50 °C underirradiation with 6 × 5 W blue LEDs (λmax = 455 nm). After 24 or 36 h, the resulting mixture wasfiltered through a 2 cm thick pad of silica, and the silica was washed with dichloromethane (DCM)(50 mL). The filtrate was collected and the solvent was removed in vacuo. The crude residue waspurified by silica gel flash column chromatography to provide the target product (2). (Note: Thereaction was very sensitive to moisture, and the yields sharply decreased to less than 5% when0.01 equivalent of H2O was added to the reaction system).
References:
Jiang, Min;Yang, Haijun;Jin, Yunhe;Ou, Lunyu;Fu, Hua [Synlett,2018,vol. 29,# 12,p. 1572 - 1577] Location in patent:supporting information
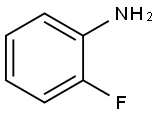
348-54-9
417 suppliers
$10.00/1g

348-52-7
292 suppliers
$6.00/5g
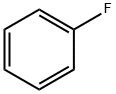
462-06-6
417 suppliers
$10.00/5g

348-52-7
292 suppliers
$6.00/5g
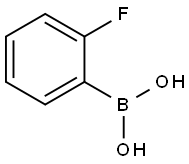
1993-03-9
358 suppliers
$8.00/1g

348-52-7
292 suppliers
$6.00/5g
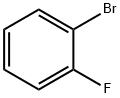
1072-85-1
451 suppliers
$5.00/10g

348-52-7
292 suppliers
$6.00/5g