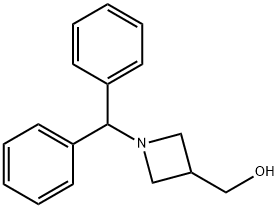
1-(DIPHENYLMETHYL)-3-(HYDROXYMETHYL)AZETIDINE synthesis
- Product Name:1-(DIPHENYLMETHYL)-3-(HYDROXYMETHYL)AZETIDINE
- CAS Number:72351-36-1
- Molecular formula:C17H19NO
- Molecular Weight:253.34

53871-06-0
95 suppliers
$38.00/1 g

72351-36-1
84 suppliers
$45.00/250mg
Yield: 100%
Reaction Conditions:
Stage #1:1-(diphenylmethyl)-3-methoxycarbonylazetidine with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 3.66667 h;
Stage #2: with sodium hydroxide;water in tetrahydrofuran at 0; for 0.333333 h;
Steps:
13
To an anhydrous tetrahydrofuran solution (100 mL) of lithium aluminum hydride (1.14 g, 29.93mmol), a tetrahydrofuran solution (50 mL) of 1-benzhydrylazetidin-3-carboxylic acid methyl ester (Oakwood Products, Inc.: Code No. W07L) (2.00 g, 7.16 mmol) was added dropwise over 10 minutes under ice cooling and stirring, and the reaction mixture was stirred for 3.5 hours. While the reaction mixture was stirred again under ice cooling, water (1.14 mL), a 4N-aqueous solution of sodium hydroxide (1.14 mL) and water (4.50 mL) were added to the reaction liquor, and the mixture was stirred for 20 minutes. Precipitated insoluble matter was removed through separation by filtration, and the obtained mother liquor was concentrated under reduced pressure to obtain the title compound (1.97 g, over yield) as an oily matter. NMR(CDCl3)δ:2.52-2.62(1H,m)3.00-3.07(2H,m)3.24(2H,t,J=7.5Hz),3.68-3.77(1H,m),3.80(2H,d,J=5.1Hz),4.34(1H,s),7.13-7.42(11H,m). MS (ESI)m/z:254 (M++1).
References:
Daiichi Sankyo Company, Limited EP1961750, 2008, A1 Location in patent:Page/Page column 78-79
36476-87-6
194 suppliers
$10.00/1g

72351-36-1
84 suppliers
$45.00/250mg
18621-17-5
378 suppliers
$5.00/1g

72351-36-1
84 suppliers
$45.00/250mg
33301-41-6
227 suppliers
$13.50/250mg

72351-36-1
84 suppliers
$45.00/250mg

36476-86-5
184 suppliers
$6.00/1g

72351-36-1
84 suppliers
$45.00/250mg