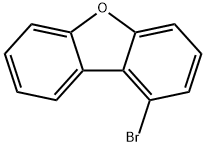
1-bromodibenzo[b,d]furan synthesis
- Product Name:1-bromodibenzo[b,d]furan
- CAS Number:50548-45-3
- Molecular formula:C12H7BrO
- Molecular Weight:247.09
1-Bromodibenzofuran organic synthesis intermediate and pharmaceutical intermediate, which can be used in laboratory research and development process and chemical and pharmaceutical synthesis process. It is prepared by stirring reaction of 6'-bromo-2'-fluorobiphenyl-2-ol and sodium hydride.
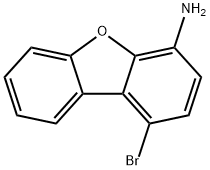
50548-39-5
22 suppliers
inquiry
![1-bromodibenzo[b,d]furan](/CAS/GIF/50548-45-3.gif)
50548-45-3
183 suppliers
$6.00/100mg
Yield:50548-45-3 61.4%
Reaction Conditions:
Stage #1:1-bromodibenzo[b,d]furan-1-amine with sulfuric acid in water;acetonitrile at -5 - 15; for 0.5 h;Inert atmosphere;
Stage #2: with isopentyl nitrite in water;acetonitrile at -5 - -3; for 1 h;
Stage #3: with hypophosphorous acid in methanol;water;acetonitrile at -2 - 20;
Steps:
1.1-4 Synthesis of Intermediate A
Under an argon atmosphere, 2,698 g (27.5 mmol) of concentrated sulfuric acid was dropped at -5 to 15° C. to a solution of 721 g (2.75 mol) of the intermediate A-3 synthesized in (1-3) in 3.6 L of acetonitrile and 3.6 L of water, followed by stirring at -5° C. for 30 min. Then, while the temperature was maintained at -3° C. or less, 483 g (4.13 mol) of isoamyl nitrite was dropped to the obtained solution, followed by stirring at -5° C. for 1 h. Then, to the solution, 908 g (13.8 mol) of hypophosphorous acid was dropped while kept at -2° C. or less. Next, to the solution, 3 L of methanol was added, followed by stirring at room temperature overnight while reaction was performed. To the reaction solution, toluene was added, and through liquid separation, a toluene layer was washed with saturated saline. The obtained residue was purified with silica gel column chromatography to obtain 418 g of an intermediate A. The yield was 61.4%.
References:
IDEMITSU KOSAN CO., LTD.;Ito, Hirokatsu;Haketa, Tasuku;Kudo, Yu;Takahashi, Yusuke US2020/317653, 2020, A1 Location in patent:Paragraph 0265; 0272-0273
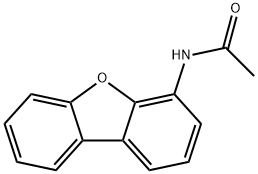
50548-37-3
4 suppliers
inquiry
![1-bromodibenzo[b,d]furan](/CAS/GIF/50548-45-3.gif)
50548-45-3
183 suppliers
$6.00/100mg
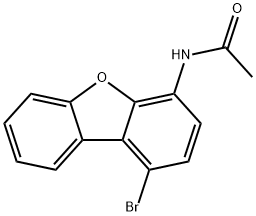
50548-38-4
5 suppliers
inquiry
![1-bromodibenzo[b,d]furan](/CAS/GIF/50548-45-3.gif)
50548-45-3
183 suppliers
$6.00/100mg
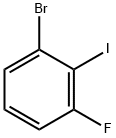
450412-29-0
163 suppliers
$16.00/1g
![1-bromodibenzo[b,d]furan](/CAS/GIF/50548-45-3.gif)
50548-45-3
183 suppliers
$6.00/100mg
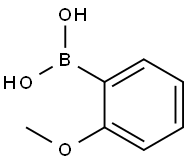
5720-06-9
360 suppliers
$5.00/1g
![1-bromodibenzo[b,d]furan](/CAS/GIF/50548-45-3.gif)
50548-45-3
183 suppliers
$6.00/100mg