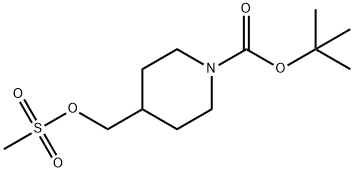
1-BOC-4-METHANESULFONYLOXYMETHYL-PIPERIDINE synthesis
- Product Name:1-BOC-4-METHANESULFONYLOXYMETHYL-PIPERIDINE
- CAS Number:161975-39-9
- Molecular formula:C12H23NO5S
- Molecular Weight:293.38
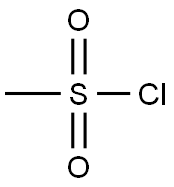
124-63-0
6 suppliers
$10.00/5g
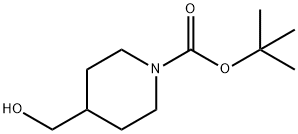
123855-51-6
412 suppliers
$6.00/5g

161975-39-9
124 suppliers
$12.00/250mg
Yield:161975-39-9 100%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 5; for 1.5 h;
Steps:
150.1 2-Fluoroethyl 4-[({6-[4-(methylsulfonyl)phenyl]-3-pyridinyl}oxy)methyl]-1-piperidinecarboxylate
A mixture of N-Boc-4-piperidinemethanol (1.0 g, 4.51 mmol), Et3N (1.30 mL, 9.01 mmol) in CH2Cl2 (20 mL) at 0° C. was treated dropwise with methanesulfonyl chloride (0.39 mL, 4.96 mmol). The reaction mixture was stirred at 0° C. to 5° C. for 1.5 h, was diluted with CH2Cl2 (100 mL) and washed with 1M NaH2PO4 (50 mL×2) and brine (25 mL). The CH2Cl2 layer was dried over Na2SO4, filtered, and the filtrate was concentrated to a brown oil, which solidified upon cooling to give 1.35 g (100%) of 1,1-dimethylethyl 4-{[(methylsulfonyl)oxy]methyl}-1-piperidinecarboxylate as a brown solid, which was used without further purification. 1H NMR (400 MHz, CDCl3): δ 4.20-4.10 (m, 2H), 4.04 (d, 2H, J=6.5 Hz), 2.99 (s, 3H), 2.75-2.60 (m, 2H), 1.95-1.85 (m, 1H), 1.75-1.65 (m, 2H), 1.43 (s, 9H), 1.30-1.10 (m, 2H).
References:
US2010/29650,2010,A1 Location in patent:Page/Page column 72

123855-51-6
412 suppliers
$6.00/5g

161975-39-9
124 suppliers
$12.00/250mg

123855-51-6
412 suppliers
$6.00/5g
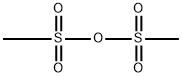
7143-01-3
274 suppliers
$19.00/5g

161975-39-9
124 suppliers
$12.00/250mg
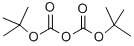
24424-99-5
835 suppliers
$13.50/25G

161975-39-9
124 suppliers
$12.00/250mg
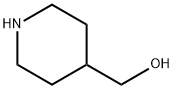
6457-49-4
386 suppliers
$18.00/25g

161975-39-9
124 suppliers
$12.00/250mg