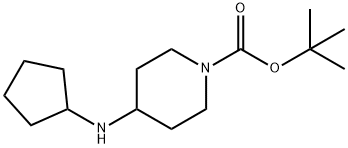
1-BOC-4-CYCLOPENTYLAMINO-PIPERIDINE synthesis
- Product Name:1-BOC-4-CYCLOPENTYLAMINO-PIPERIDINE
- CAS Number:812690-40-7
- Molecular formula:C15H28N2O2
- Molecular Weight:268.39
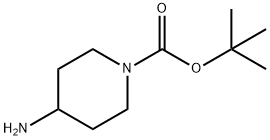
87120-72-7
20 suppliers
$6.00/1g
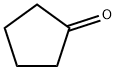
120-92-3
413 suppliers
$11.00/25g

812690-40-7
26 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
Stage #1: 1-(tert-butoxycarbonyl)-4-aminopiperidine;cyclopentanone in methanol at 20; for 18 h;
Stage #2: with sodium tetrahydroborate for 6 h;
Steps:
34 Preparation 34tert-Butyl 4-(cvclopentylamino)piperidine-1 -carboxvlate
A solution of cyclopentanone (139mg, 1.65mmol) and ferf-butyl 4-amino-piperidine-1-carboxylate (SOOmg, 1.5mmol) in methanol (6ml) was stirred at room temperaturefor 18 hours. Sodium borohydride (113mg, S.Ommol) was added portionwise, and thereaction mixture was stirred for a further 6 hours. The mixture was partitionedbetween saturated aqueous sodium bicarbonate solution and ethyl acetate and thelayers were separated. The aqueous layer was extracted with further ethyl acetate(x3) and the combined organic extracts were dried (MgSO4) and evaporated underreduced pressure. The residue was purified by column chromatography on silica gelusing an elution gradient of pentane:ethyl acetate:methanol (50:50:0 to 0:90:10) togive the title compound as a colourless oil, 310mg.1H-nmr (CDCI3, 400MHz) 8:1.22-1.33 (m, 4H), 1.45 (s, 9H), 1.49-1.58 (m, 2H), 1.64-1.73 (m, 2H), 1.84-1.94 (m, 4H), 2.63-2.70 (m, 1H), 2.71-2.80 (brm, 2H), 3.21 (q,1H), 4.05 (farm, 2H).LRMS: m/z APCI+ 269 [MH+]
References:
WO2004/111003,2004,A1 Location in patent:Page 63-64
79099-07-3
543 suppliers
$5.00/5g
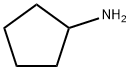
1003-03-8
222 suppliers
$10.00/1g

812690-40-7
26 suppliers
$45.00/50mg