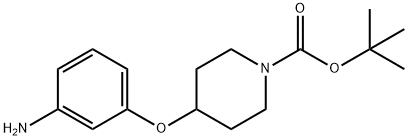
1-BOC-4-(3-AMINOPHENOXY)PIPERIDINE synthesis
- Product Name:1-BOC-4-(3-AMINOPHENOXY)PIPERIDINE
- CAS Number:790667-68-4
- Molecular formula:C16H24N2O3
- Molecular Weight:292.37
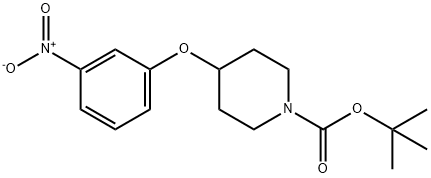
586412-88-6
12 suppliers
inquiry

790667-68-4
23 suppliers
$110.00/0.25g
Yield:790667-68-4 100%
Reaction Conditions:
with hydrogen;nickel in ethanol at 20; under 37503.8 Torr;
Steps:
B.B1
tert-Butyl 4-(3-nitrophenoxy)piperidine-1-carboxylate (prepared as de- scribed in WO-A-2003/068760) (10.764 g; 33.39 mmol) is dissolved in 430 ml of ethanol, Raney nickel (7.2 g) is then added and the reaction medium is placed under hydrogen (pressure: 50 bar) at room temperature overnight. The reaction medium is filtered through Celite and evaporated to dryness to give quantitatively (9.76 g) the expected product in the form of a beige-coloured oil. TLC (97/3 dichloromethane/methanol): Rf = 0.53 Yld: quantitative 1H NMR (300 MHz, chloroform-D) No. ppm: 1.5 (s, 9 H); 1.7 (m, 2 H); 1.9 (m, 2 H) ; 3.3 (m, 2 H) ; 3.7 (m, 2 H) ; 4.4 (m, 1 H) ; 6.3 (m, 3 H); 7.1 (t, J = 8.0 Hz, 1 H); 7.3 (s, 2 H).
References:
WO2005/121091,2005,A1 Location in patent:Page/Page column 20-21
![1-Piperidinecarboxylic acid, 4-[3-[(diphenylmethylene)amino]phenoxy]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/790667-69-5.gif)
790667-69-5
0 suppliers
inquiry

790667-68-4
23 suppliers
$110.00/0.25g
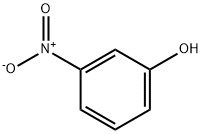
554-84-7
8 suppliers
$12.00/5g

790667-68-4
23 suppliers
$110.00/0.25g
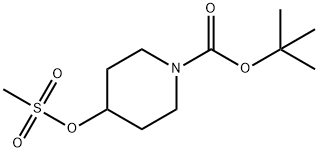
141699-59-4
233 suppliers
$6.00/1g

790667-68-4
23 suppliers
$110.00/0.25g
79099-07-3
543 suppliers
$5.00/5g

790667-68-4
23 suppliers
$110.00/0.25g