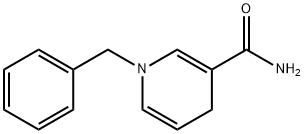
1-BENZYL-1,4-DIHYDRONICOTINAMIDE synthesis
- Product Name:1-BENZYL-1,4-DIHYDRONICOTINAMIDE
- CAS Number:952-92-1
- Molecular formula:C13H14N2O
- Molecular Weight:214.26
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

952-92-1
58 suppliers
$25.00/250mg
Yield: 73%
Reaction Conditions:
with sodium dithionite;sodium carbonate in water at 20; for 0.25 h;Darkness;Inert atmosphere;
Steps:
5.2 synthesis of 1-benzyl-1,4-dihydropyridine-3-carboxamide
To a solution of 1 -benzylpyridinium-3 carboxamide iodide (1 g, 3.41 mmol) in H20 (15 mL) was added sodium carbonate (1.44 g, 13.64 mmol) and sodium dithionite (2.65 g, 12.96 mmol) at room temperature. The resulting solution was stirred under nitrogen in darkness for 15 mn. The reaction mixture was then extracted with dichioromethane. The combined organic phases were dried overNaSO, filtered and evaporated under reduced pressure at room temperature to afford N-benzyl4 ,4- dihydronicotinamide (BNAH) (0.530 g, 73%).1HNMR(CDCl3, 300 MHz, 298K, 8 ppm): 7.34 (m, 5 H), 7.26 (s, I H), 5.75 (d, 1 H,J= 8,10Hz),5.38 (s, 2 H), 4.76 (m, 1 H), 4.30 (s, 2 H), 3.19 (s, 2 H).13C NMR (CDC13, 75.5 MHz, 298 K, 8 ppm): 140.15, 137.37, 129.09, 128.95, 127.92, 127.29,103.34, 98.75, 57.52, 22.97.
References:
INSA (INSTITUT NATIONAL DES SCIENCES APPLIQUéES) DE ROUEN;CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (CNRS);UNIVERSITE DE ROUEN;VFP THéRAPIES;MARSAIS, Francis;LEVACHER, Vincent;PAPAMICAEL, Cyril;BOHN, Pierre;PEAUGER, Ludovic;GEMBUS, Vincent;LE FUR, Nicolas;DUMARTIN-LEPINE, Marie-Laurence WO2014/114742, 2014, A1 Location in patent:Page/Page column 37; 38
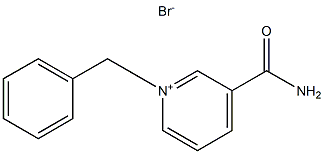
13076-43-2
14 suppliers
inquiry

952-92-1
58 suppliers
$25.00/250mg
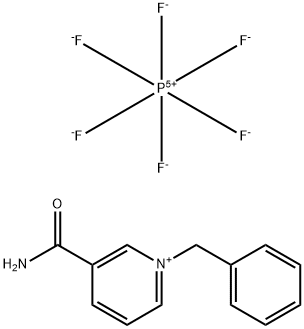
90832-35-2
0 suppliers
inquiry

952-92-1
58 suppliers
$25.00/250mg
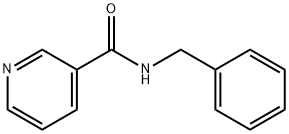
2503-55-1
10 suppliers
inquiry

952-92-1
58 suppliers
$25.00/250mg
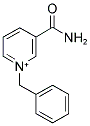
16183-83-8
2 suppliers
inquiry

952-92-1
58 suppliers
$25.00/250mg