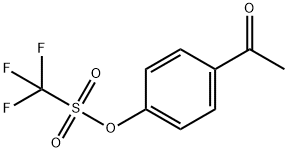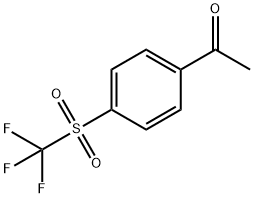
1-(4-(trifluoromethylsulfonyl)phenyl)ethanone synthesis
- Product Name:1-(4-(trifluoromethylsulfonyl)phenyl)ethanone
- CAS Number:432-86-0
- Molecular formula:C9H7F3O3S
- Molecular Weight:252.21
Yield:432-86-0 92%
Reaction Conditions:
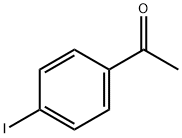
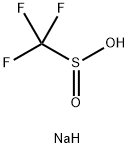
Stage #1: 4-Iodoacetophenonewith (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;tert-butylammonium hexafluorophosphate(V);caesium carbonate;Aminoiminomethanesulfinic acid in N,N-dimethyl-formamide at 90; for 2.5 h;Inert atmosphere;
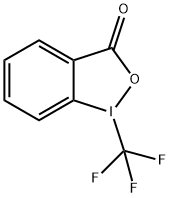
Stage #2: Togni's reagent II in N,N-dimethyl-formamide at 25; for 1 h;Inert atmosphere;
Steps:
36 Preparation of compound 5d:
Add 4'-iodoacetophenone (49.2mg, 0.2mmol), thiourea dioxide (32.4mg, 0.3mmol), PdCl2dppf (11.0mg, 0.015mmol), nBu4NPF6 (38.7mg) to the reaction tube under a nitrogen atmosphere , 0.1mmol), Cs2CO3 (130.4mg, 0.4mmol) and redistilled solvent DMF (2mL) were reacted at 90°C for 2.5 hours. After cooling to room temperature, Togni's Reagent II reagent (69.6 mg, 0.22 mmol) was added to the system, and stirred at room temperature (25° C.) for 1 hour. After the reaction, water was added to the system to quench the reaction, extracted with ethyl acetate four times, the organic phases were combined and then washed with saturated brine once, dried over anhydrous Na2SO4, concentrated under reduced pressure, and purified by column chromatography (PE/EA =10:1, Rf=0.2) 46.4mg of yellow solid 5d was obtained, and the yield was 92%.
References:
CN113527158,2021,A Location in patent:Paragraph 0175-0178
31218-75-4
8 suppliers
inquiry

432-86-0
4 suppliers
inquiry
![Benzene, 1-ethyl-4-[(trifluoromethyl)thio]-](/CAS/20200119/GIF/458-99-1.gif)
458-99-1
0 suppliers
inquiry

432-86-0
4 suppliers
inquiry