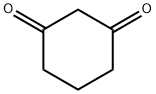
1,3-Cyclohexanedione synthesis
- Product Name:1,3-Cyclohexanedione
- CAS Number:504-02-9
- Molecular formula:C6H8O2
- Molecular Weight:112.13
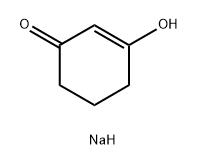
57910-97-1
0 suppliers
inquiry

504-02-9
561 suppliers
$5.00/10g
Yield:504-02-9 96.43%
Reaction Conditions:
with hydrogenchloride in water at 15 - 20;
Steps:
1.1-1.4; 2-13 (1) Hydrogenation, acidification and crystallization
Resorcinol (99%, 0.535mol) was neutralized with sodium hydroxide aqueous solution (15%, 0.589mol), and then hydrogenated at 5MPa and 120°C to obtain a reaction solution containing the compound represented by the formula (30% , 0.535mol), and the reaction solution was acidified with hydrochloric acid (30%, 0.562mol) at 15-20°C to obtain 1,3-cyclohexanedione acidified solution, and then the 1,3-cyclohexane The dione acidified liquid was cooled at 5°C to crystallize and filtered and dried to obtain 1,3-cyclohexanedione product (99%, 0.426mol) and an acidified mother liquor containing 1,3-cyclohexanedione. (2) Extraction of 1,3-cyclohexanedioneFirst extraction: At room temperature, add 75g trioctylamine (that is, tri-n-octylamine) and 125g kerosene to a 1L reaction flask to obtain a complexing agent mixture. Under stirring, add the 1, obtained in step (1), The acidified mother liquor of 3-cyclohexanedione was stirred at room temperature for 5 minutes, and then stood still for liquid separation. The obtained upper kerosene phase containing the trioctylamine complex of 1,3-cyclohexanedione is used to dissociate 1,3-cyclohexanedione.Second extraction: the lower aqueous phase obtained from the first extraction is added to the prepared complexing agent mixture with 75g trioctylamine dissolved in 125g kerosene, stirred for 5 minutes, and left to stand for liquid separation. The upper trioctylamine kerosene phase was used for the first extraction of the next batch.The third extraction: the lower aqueous phase obtained from the second extraction is added to the prepared complexing agent mixture with 75 g of trioctylamine dissolved in 125 g of kerosene, stirred for 5 minutes, and left to stand for liquid separation. Separate the lower aqueous phase (1,3-cyclohexanedione content 0.0007%, COD: 48ppm); the upper trioctylamine kerosene phase is used for the second extraction of the next batch.(3) Dissociation of trioctylamine complex of 1,3-cyclohexanedioneAt room temperature, add the kerosene phase containing the trioctylamine complex of 1,3-cyclohexanedione obtained in the first extraction into the 1L reaction flask, and heat to 45-50°C, and add the kerosene phase obtained in step (1) Reaction solution (30%, 0.535mol), add 30% sodium hydroxide dropwise to adjust pH=12-13, stir for 2 hours, stand still for liquid separation, separate the lower layer of 1,3-cyclohexanedione sodium salt aqueous solution; upper layer The trioctylamine kerosene phase is used for the third extraction of the next batch of 1,3-cyclohexanedione acid jellyfish liquor.(4) Crystal of 1,3-cyclohexanedioneAdjust the pH of the lower layer 1,3-cyclohexanedione sodium salt aqueous solution = 1.5 to crystallize 1,3-cyclohexanedione, filter and dry the filter cake to obtain 1,3-cyclohexanedione product (99.5%, 0.5174 mol), the yield was 96.23%.
References:
CN111302909,2020,A Location in patent:Paragraph 0068-0095
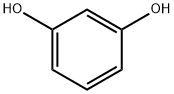
108-46-3
767 suppliers
$10.00/10g

504-02-9
561 suppliers
$5.00/10g
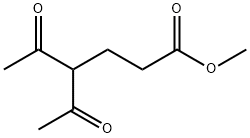
13984-53-7
35 suppliers
$60.00/50mg

504-02-9
561 suppliers
$5.00/10g
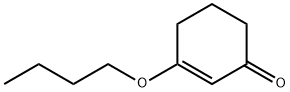
16493-04-2
32 suppliers
$87.00/1g

504-02-9
561 suppliers
$5.00/10g
![7-OXABICYCLO[4.1.0]HEPTAN-2-ONE](/CAS/GIF/6705-49-3.gif)
6705-49-3
74 suppliers
$18.00/250mg

504-02-9
561 suppliers
$5.00/10g