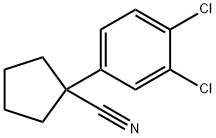
1-(3,4-DICHLOROPHENYL)CYCLOPENTANECARBONITRILE synthesis
- Product Name:1-(3,4-DICHLOROPHENYL)CYCLOPENTANECARBONITRILE
- CAS Number:213755-91-0
- Molecular formula:C12H11Cl2N
- Molecular Weight:240.13
Yield:213755-91-0 86%
Reaction Conditions:
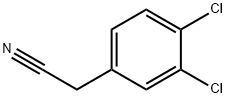
Stage #1: 3,4-dichloro-benzeneacetonitrilewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0;Inert atmosphere;
Stage #2: 1,4-dibromo-butane in N,N-dimethyl-formamide;mineral oil at 20;
Steps:
120.1 Step 1: Synthesis of ( 13-,4-dichlorophenyl)cyclopentanecarbonitrile
In a three necked round bottomed flask equipped with a thermometer,under nitrogen, a suspension of sodium hydride (60%, 591 mg, 14.8 mmol) in dry DMF (16 mL) was stirred at 0 °C. A solution of (3,4-dichlorophenyl)acetonitrile (1.00 g, 5.38 mmol) in dry DMF (6 mL) was added dropwise and the mixture was stirred at 0 °C for 30 min. A solution of 1,4- dibromobutane (1.74 g, 8.06 mmol) in dry DMF (6 mL) was added dropwise and the mixture was stirred at rt for 3 h. The mixture was stirred at 0 °C and half saturated aqueous NH4CI (70 mL) was added dropwise. EtOAc was added and the mixture was stirred at rt for 10 min. The aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried over sodium sulfate, filtered and concentrated. The crude was purified by flash chromatography on silica gel using a gradient of EtOAc in heptane from 1% to 5% to afford the title compound as a colorless liquid (1.12 g, 86% yield, ti = 1.01 min). LCMS (Method F): m/z found 294.0 [M+H]+;1H-NMR (DMSO-d6, 400 MHz): δ (ppm) 7.77 (d, J=2.3 Hz, 1H), 7.70 (d, J=8.5 Hz, 1H), 7.53 (dd, J=8.5, 2.3 Hz, 1H), 2.49 - 2.39 (m, 2H), 2.14 - 2.02 (m, 2H), 1.95 - 1.85 (m, 3H).
References:
WO2022/167866,2022,A1 Location in patent:Page/Page column 276-277