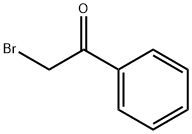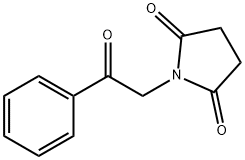
1-(2-oxo-2-phenylethyl)pyrrolidine-2,5-dione synthesis
- Product Name:1-(2-oxo-2-phenylethyl)pyrrolidine-2,5-dione
- CAS Number:24246-87-5
- Molecular formula:C12H11NO3
- Molecular Weight:217.22
Yield: 71%
Reaction Conditions:
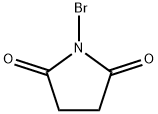
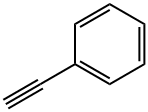
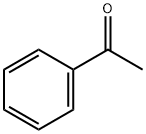
Stage #1:N-Bromosuccinimide;phenylacetylene with water at 80; for 1 h;
Stage #2: with 1,8-diazabicyclo[5.4.0]undec-7-ene in acetone at 20; for 2 h;Mechanism;Reagent/catalyst;Solvent;Temperature;
Steps:
Representative procedure for the synthesis of α-imino ketones from alkynes
To a stirred solution of phenylacetylene 1a (102.12 mg, 1.0 mmol) and H2O (1.0 mL) in a vial (4.0 mL) were added N-Bromosuccinimide (356 mg, 2.0 mmol). Upon sealing by a septum cap and stirring at 80 °C for 1 h, the reaction mixture was then cooled to room temperature. Subsequently, acetone (1 mL) and DBU (3.0 mmol) were introduced into the reaction mixture, and the stirring was continued at room temperature for additional 2 h. After completion of reaction as monitored by TLC analysis, acetone was evaporated in vaccuo and crude product was poured into water and then extracted with CH2Cl2 (3×10 mL). The organic phase was washed with water (3×10 mL), dried over Na2SO4, filtered and concentrated in vaccuo.The crude product was purified by a short-pad silica gel column chromatography with petroleum ether as eluent to give compound 2a as a white solid (154.1 mg, 71% yield). Table 2, entry 1 2a Yield: 71% (154 mg); White solid. m.p. 160-151 °C. 1H NMR (400 MHz, CDCl3) δ 2.88 (s, 4H), 4.96 (s, 2H), 7.51 (t, 2H), 7.61 (t, 1H), 7.91 (d, 2H); 13C NMR (100 MHz, CDCl3) δ 28.4, 44.7, 128.1, 128.9, 134.3, 176.7, 190.2.
References:
Wei, Ting;Zeng, Yongming;He, Wei;Geng, Lili;Hong, Liang [Chinese Chemical Letters,2019,p. 383 - 385] Location in patent:supporting information
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

24246-87-5
7 suppliers
inquiry