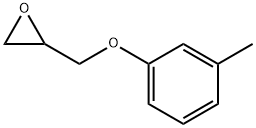
1,2-EPOXY-3-(3-METHYLPHENOXYPROPANE) synthesis
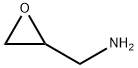
- Product Name:1,2-EPOXY-3-(3-METHYLPHENOXYPROPANE)
- CAS Number:2186-25-6
- Molecular formula:C10H12O2
- Molecular Weight:164.2
Yield:2186-25-6 100%
Reaction Conditions:
with potassium carbonate in butanone at 70 - 86; for 66 h;
Steps:
1.5 General Method for Preparation of the Oxiranes (Examples 1.5 to 1.7; Other Oxiranes Used are Commercially Available)
General procedure: General Method for Preparation of the Oxiranes (Examples 1.5 to 1.7; Other Oxiranes Used are Commercially Available) (0158) 1 equivalent of phenol and 2.4 equivalents of potassium carbonate were initially charged in 2-butanone. Then 3 equivalents of epibromohydrin were added gradually at room temperature. The amount of 2-butanone corresponded to 50 percent by weight of the total amount. There was a preliminary check of whether the phenol dissolves sufficiently in 2-butanone. The potassium carbonate suspension was then boiled under reflux. (0159) Once full conversion had been attained, was checked by 1H NMR spectroscopy (see below for statement of time), the potassium carbonate was filtered off and the mixture was concentrated on a rotary evaporator. This gave the liquid, clear products, some of which were coloured, without further workup. The yield based on the phenol used was quantitative. RRN 27Example 1.5 2-[(3-methylphenoxy)methyl]oxirane (0160) Reactants: 11.9 g m-cresol (0161) 45.2 g epibromohydrin (0162) 36.4 g potassium carbonate (0163) 93.5 g 2-butanone (0164) Conditions: 16.5 h at 86° C. and 49.5 hours at 70° C. (0165) 1H NMR (CDCl3, 400 MHz): δ (1H)=7.18 (t, 1H), 6.77 (d; 1H), 6.68-6.75 (m, 2H), 4.18 (dd, 3.95 (dd, 1H), 3.24 (m, 1H), 2.89 (dd, 1H), 2.73 (dd, 1H), 2.32 (s, 3H).
References:
US2017/45816,2017,A1 Location in patent:Paragraph 0160; 0161; 0162; 0163; 0164; 0165
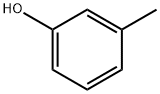
108-39-4
488 suppliers
$13.00/25g

2186-25-6
36 suppliers
inquiry

108-39-4
488 suppliers
$13.00/25g

106-89-8
754 suppliers
$9.00/10g

2186-25-6
36 suppliers
inquiry
42865-04-3
2 suppliers
inquiry