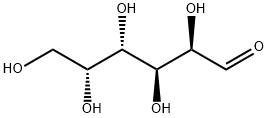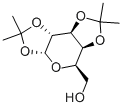
1,2:3,4-Di-O-isopropylidene-D-galactopyranose synthesis
- Product Name:1,2:3,4-Di-O-isopropylidene-D-galactopyranose
- CAS Number:4064-06-6
- Molecular formula:C12H20O6
- Molecular Weight:260.28
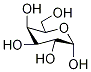
3646-73-9
30 suppliers
$403.00/5mg
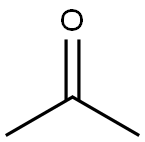
67-64-1
0 suppliers
$17.30/10ml

4064-06-6
232 suppliers
$11.00/1g
Yield:4064-06-6 75.6%
Reaction Conditions:
with sulfuric acid;zinc(II) chloride at 20; for 4 h;Inert atmosphere;
Steps:
2.3 Synthesis of 1,2;3,4-di-O-isopropylidene-D-galactose (IpGal) (2)
D-galactose (7g, 38.8mmol) and anhydrous ZnCl2 (7.14g, 52.4mmol) were added to acetone (150mL) followed by H2SO4 (0.84mL) dropwise to the solution. It was allowed to react at room temperature for 4h under nitrogen atmosphere until the starting material completely disappeared. The solution was neutralized with saturated Na2CO3 solution after the reaction. After filtration, the solvent was removed under reduced pressure on a rotary evaporator. It was further extracted with diethyl etherand dried over anhydrous Na2SO4. The organic solvent was evaporated, and the product 2 was obtained as a pale-yellow viscous oil in 75.6% yield which was used for the next step without further purification. 1H NMR (400MHz, CDCl3) δ 5.57 (1H, d, J=5.2Hz, anomeric proton CH), 4.62 (1H, dd, J=8.0Hz, 2.4Hz, CH), 4.34 (1H, dd, J=5.2Hz, 2.4Hz, CH), 4.28 (1H, dd, J=8.0Hz, 1.7Hz, CH), 3.87-3.75 (3H, m, CH+CH2), 2.14 (1H, m, OH), 1.45 (3H, s, CH3), 1.33 (3H, s, CH3), 1.25 (6H, s, 2CH3); 13C NMR (100MHz, CDCl3) δ 108.9, 96.5, 71.8, 71.0, 70.8, 68.4, 62.5, 26.2, 26.1, 25.1, 24.5; FTIR (ν ν (cm-1) 3489 (br, O-H stretch), 2985, 2933 (C-H stretch), 1069 (C-O stretch).
References:
Goel, Shubhra;Jacob, Josemon [Reactive and functional polymers,2020,vol. 157,art. no. 104766]
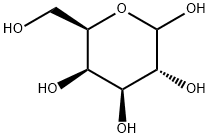
10257-28-0
79 suppliers
$72.79/500 mg
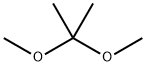
77-76-9
399 suppliers
$9.00/5ml

4064-06-6
232 suppliers
$11.00/1g
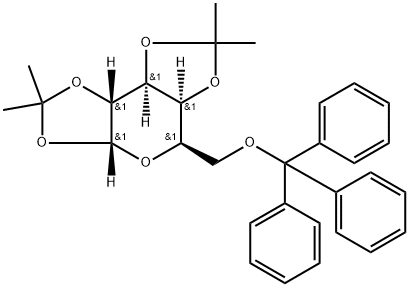
76951-66-1
3 suppliers
inquiry

4064-06-6
232 suppliers
$11.00/1g

10257-28-0
79 suppliers
$72.79/500 mg

67-64-1
0 suppliers
$17.30/10ml

4064-06-6
232 suppliers
$11.00/1g