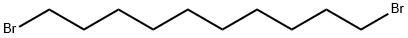
1,10-Dibromodecane synthesis
- Product Name:1,10-Dibromodecane
- CAS Number:4101-68-2
- Molecular formula:C10H20Br2
- Molecular Weight:300.07
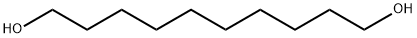
112-47-0
325 suppliers
$6.00/25g
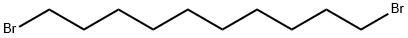
4101-68-2
306 suppliers
$6.00/10g
Yield:4101-68-2 91%
Reaction Conditions:
with hydrogen bromide in octane;water at 145; for 7 h;Dean-Stark;
Steps:
General procedure
General procedure: A one-neck rb flask was charged with diol (1equiv), 48% aq HBr (~3 equiv/OH), octane (~7:1 v/w ratio vs diol), fitted tothe fractionating column/Dean-Stark trap, and heated inan oil bath (145-150 °C) w/rapid magnetic stirring. The aqueous (lower) layer of the initialazeotrope condensate (bp 89-92 °C) was tapped offuntil about half of the theoretical amount of H2O had been collected;the azeotrope temp (still head) then began to rise. The condenser was set to total reflux forseveral h, reopened, and aq material collected for 1h more (head temp 96-100°C). The final volume of aq distillatewas 90-100+% of theory (higher-boiling distillate contained up to 24% HBr). When the (pale tan) octane phase containedboth dibromide and bromoalkanol (6band 6c), washing with cold 85% v/v H2SO4 (10 mL,then 5 mL) removed all color and all bromoalkanol. For all three dibromides (3b, 4b, 6b) the neutralized octane solutionwas stripped of solvent (vigreux column, reduced pressure), and the essentiallypure residue (1H NMR) was kugelrohr distilled. A trace of 4-methyltetrahydropyran was foundin 4b before distillation.
References:
Mekala, Shekar;Hahn, Roger C. [Tetrahedron Letters,2015,vol. 56,# 4,p. 630 - 632] Location in patent:supporting information
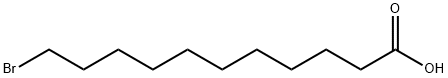
2834-05-1
215 suppliers
$6.00/5g
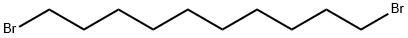
4101-68-2
306 suppliers
$6.00/10g
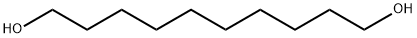
112-47-0
325 suppliers
$6.00/25g

53463-68-6
201 suppliers
$6.00/1g
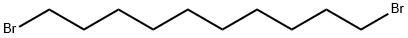
4101-68-2
306 suppliers
$6.00/10g
1647-16-1
138 suppliers
$12.00/5g
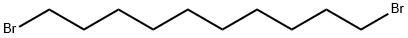
4101-68-2
306 suppliers
$6.00/10g
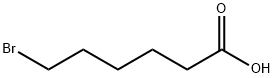
4224-70-8
350 suppliers
$7.00/5g
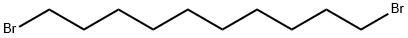
4101-68-2
306 suppliers
$6.00/10g