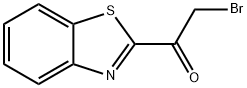
1-(1,3-Benzothiazol-2-yl)-2-bromo-1-ethanone synthesis
- Product Name:1-(1,3-Benzothiazol-2-yl)-2-bromo-1-ethanone
- CAS Number:54223-20-0
- Molecular formula:C9H6BrNOS
- Molecular Weight:256.12
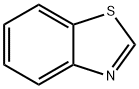
95-16-9
404 suppliers
$14.14/10gm:
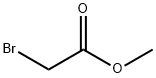
96-32-2
344 suppliers
$15.00/5g

54223-20-0
54 suppliers
$41.00/250mg
Yield:54223-20-0 27%
Reaction Conditions:
Stage #1: 1,3-Benzothiazolewith n-butyllithium in hexanes;diethyl ether at -78; for 0.25 h;
Stage #2: bromoacetic acid methyl ester in hexanes;diethyl ether at -78; for 0.25 h;
Steps:
76A
Preparation 76A; 1-(be?zordlthiazol-2-yl>-2-bromoethano?e; n-Butyllithium in hexa?es (6.21 ml. of 2.5 M) was added dropwise to a stirred -780C solution of benzothiazole (2.00 g, 14.8 mmol) in ether (20 mL). After 15 min, methyl bromoacetatθ (2.4 g, 15.5 mmol) was added in one portion at - 78 0C giving a suspension, which was stirred at -78 0C for 15 min. Acetic acid (1.8 g, 31 mmol) was added at - 78 0C and the mixture was warmed to RT. Ether (20 mL) and water were added. The organic layer was separated, dried, and concentrated. The resulting oily solid was dissolved in hot isopropyl ether and the suspension filtered. The filtrate was evaporated and the residue suspended in hexa?es. The solid was filtered and dried. Yield 1.02 g, 27%, orange solid. 1H NMR (CDCI3,400 mHz) δ 8.20 (m, 1H), 8.01 (m, 1H), 7.63-7.55 (m, 2H), 4.84 (s, 2H).
References:
WO2008/4117,2008,A1 Location in patent:Page/Page column 143
32137-73-8
39 suppliers
$56.79/1g
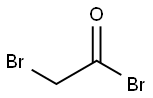
598-21-0
310 suppliers
$10.00/10g

54223-20-0
54 suppliers
$41.00/250mg
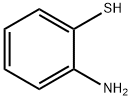
137-07-5
302 suppliers
$10.00/5g

54223-20-0
54 suppliers
$41.00/250mg