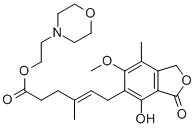
Properties and applications of Mycophenolate mofetil
Sep 14,2023
Description
Mycophenolate mofetil is also known as MMF. The FDA approved its official registration in 1995 under the trade name Cellcept. It was developed by the American company Syntex and is a semi-synthetic derivative of mycophenolic acid (MPA) isolated from the fermentation of mold Penicillin glaucum. As a reusable cytostatist, MMF has been used clinically for more than 10 years.
Properties
The formula of MMF is C23H31NO7 and it is a white or off-white crystalline powder. MMF is highly soluble in dichloromethane and can be dissolved in acetonitrile, ethyl acetate, and 0.1mol/L hydrochloric acid solution, but insoluble in water. Its synthesis is mainly divided into two steps. First, mycophenolic acid is used as raw material to undergo a chlorination reaction with thionyl chloride. 4-(2-hydroxyethyl) morpholinem was then added for esterification to obtain mycophenolate mofetil.

Mycophenolate mofetil is different from mycophenolic acid which is an active metabolite of mycophenolate mofetil. Mycophenolate mofetil is a prodrug of mycophenolic acid that after oral administration is rapidly hydrolyzed to mycophenolic acid. It is an agent that inhibits the proliferation of B and T lymphocytes through noncompetitive, reversible inhibition of inosine monophosphate dehydrogenase, a key enzyme in the de novo synthetic pathway of guanine nucleotides[1]. Another formulation of mycophenolic acid is mycophenolate sodium, which can also be administered orally.
Applications
MMF is highly safe, non-pulmonary toxic, and can act on organ transplantation and other inflammatory conditions. This drug is now a widespread immunosuppressive drug for the treatment of several conditions, including lupus nephritis, interstitial lung disease associated with systemic sclerosis, and anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis while being efficacious also as rescue therapy in various orphan diseases, including dermatomyositis and IgA-associated nephropathy. For children with frequently relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS) experiencing steroid toxicity, MMF has been recommended as a steroid-sparing [2] It is used in graft-versus-host disease (GVHD) prophylaxis and management to induce colitis. The study found that MMF-induced colitis generally presents with watery diarrhea but rarely blood may be noted in stool. Discontinuation of MMF may lead to transplant rejection.
References
[1] Bahram M, et al. Simultaneous kinetic–spectrophotometric determination of mycophenolate mofetil and mycophenolic acid based on complexation with Fe(III) using chemometric techniques. Journal of the Iranian Chemical Society, 2018; 15: 779–786.
[2] Sievers T, et al. Mycophenolate mofetil. Pharmacotherapy, 1997.
[3] Papiris S, et al. Mycophenolate mofetil as an alternative treatment in sarcoidosis. Papiris, 2019; 58: 101840.
[4] Bhat R, et al. Perspectives on Mycophenolate Mofetil in the Management of Autoimmunity. Clinical Reviews in Allergy Immunology, 2023; 65: 86–100.
- Related articles
- Related Qustion
- Mycophenolate mofetil: pharmacokinetics, mechanism of action and applications in dermatology Jan 29, 2024
Mycophenolate mofetilis can be converted to mycophenolic acid and inhibit T and B cell proliferation via depletion of guanosine nucleotides, showing promise in treating inflammatory skin diseases.
Mycophenolate mofetil
115007-34-6You may like
Mycophenolate mofetil manufacturers
- Mycophenolate mofetil
-

- $0.00 / 25Kg/Drum
- 2024-12-13
- CAS:115007-34-6
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 500kgs
- RS 61443
-
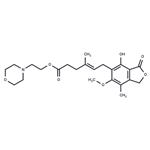
- $31.00 / 25mg
- 2024-11-19
- CAS:115007-34-6
- Min. Order:
- Purity: 99.37%
- Supply Ability: 10g
- RS 61443
-
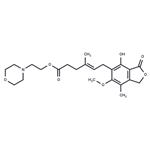
- $31.00 / 25mg
- 2024-11-19
- CAS:115007-34-6
- Min. Order:
- Purity: 99.37%
- Supply Ability: 10g