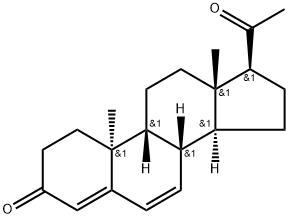
Dydrogesterone: Synthesis and Applications
Mar 13,2025
Dydrogesterone is a potent orally active progesterone receptor agonist that was developed in the 1950s and that has been widely used since the 1960s for menstrual disorders such as premenstrual syndrome , cycle irregularity, endometriosis , threatened miscarriage , and habitual miscarriage, and for postmenopausal hormone therapy . Unlike other members of the progestin family, dydrogesterone and its main active metabolite, 20α-hydroxydydrogesterone, do not have any clinically relevant agonistic or antagonistic activity on the androgen, estrogen, and glucocorticoid receptors and only mild antimineralocorticoid properties. Safety concerns owing to receptor cross-activation have precluded the use of the majority of the progestins in fertility treatment and pregnancy. Only bioidentical progesterone, 17-hydroxy-progesteronecaproate and dydrogesterone are considered to be sufficiently safe for the developing fetus.

Synthesis of Dydrogesterone
In a 100 mL three-necked flask, add 0.42 g (4.2 mmol) of cuprous chloride and 42 mL of DMF, replace with nitrogen three times, heat to 65° C., keep stirring under nitrogen protection for 1 hour, and cool to room temperature for later use. In a 500mL three-necked flask, add 28g (71.2mmol) of compound G and 280mL of dichloromethane, cool down to 0-5°C, add cuprous chloride solution, pass in the dried air, keep the gas flow rate 1L/min, react 4 After 8 hours, TLC detects that the remaining raw material is less than 2%, and it can be stopped if there is no change in the prolonged time. Add 10% sulfuric acid solution to quench, separate liquid, add 1% sulfuric acid solution to wash the organic phase, add 0.43g acetic acid to the organic phase, stir for 5 minutes, add 6% sodium chlorite solution, stir at room temperature for 30 minutes, TLC raw materials almost disappear. Sodium thiosulfate was added to quench, and the solution was separated. The organic phase was washed with 0.5% sodium hydroxide and brine in turn, the organic phase was concentrated below 50°C, and the crude product was obtained by water replacement. The crude product was dissolved in 280 mL of acetone, concentrated to a small volume, cooled to -20 °C, cooled for 2 hours, filtered, the filter cake was rinsed with ice acetone, drained, and dried in an oven at 45 °C to obtain 20 g of solid compound Dydrogesterone. The yield of Dydrogesterone is about 74.6%.[1]
Dydrogesterone for the treatment of threatened miscarriage
The objective of this systematic review was to assess whether the orally acting progestagen, dydrogesterone lowers the incidence of miscarriage in women with threatened miscarriage. A computerized search was performed in Medline, Embase, and Ovid Medline for original reports with the product name ‘Duphaston’ or ‘dydrogesterone’, and limited to clinical human data. Twenty-one reports of dydrogesterone treatment were identified with 1380 patients. Five randomized trials were identified, including 660 women who fulfilled the criteria for metaanalysis. The number of subsequent miscarriages or continuing pregnancies per randomized woman was compared in women receiving dydrogesterone compared to standard bed rest or placebo intervention. There was a 13% (44/335) miscarriage rate after dydrogesterone administration compared to 24% in control women [odds ratio for miscarriage 0.47, (CI = 0.31–0.7), 11% absolute reduction in the miscarriage rate]. The adverse and side effects were summarized in all 21 reports, and seemed to be minimal. Although all the predictive and confounding factors could not be controlled for, the results of this systematic review show a significant reduction of 47% in the odds for miscarriage when dydrogesterone is compared to standard care indicating a real treatment effect.[2]
The results of this metaanalysis of 660 patients show that the effect of dydrogesterone on the risk of miscarriage in women with threatened miscarriage appears to be substantiated. There was a statistically significant reduction in the odds ratio for miscarriage after dydrogesterone compared to standard care of 0.47 (CI = 0.31–0.7) rate. The 24% miscarriage rate in control women (78/325) was reduced to 13% (44/335) after dydrogesterone administration (11% absolute reduction in the miscarriage rate). There is consistency between the results of the five trials in that the confidence limits all overlap. There seem to be no significant side effects.
Although treatment with progestagens in general and dydrogesterone in particular are somewhat empiric, the results of this systematic review showed that dydrogesterone was associated with a reduction of 47% in the odds for miscarriage, compared to standard care and an absolute decrease in the miscarriage rate of 11%. In many parts of the world, early ultrasound, progesterone and hCG levels are not generally available. Even when available, the patient demands treatment which is risk free and decreases the chance of threatened miscarriage terminating in miscarriage. The evidence presented here suggests that the treating physician should comply with her wishes.
Dydrogesterone for treatment of dysmenorrhea
By preventing ovulation, LEP or OC suppresses the progesterone-driven proliferation of the secretory endometrium, resulting in a decrease in the visual analog scale (VAS) score.4, 5 Recently, while low-dose hormone preparations are becoming increasingly popular, treatment with low-dose hormone preparations has been suggested to be associated with a risk of venous thrombosis, and other adverse reactions. Under these circumstances, dydrogesterone (Duphaston), a hormone preparation available since 1965 for the treatment of not only dysmenorrhea but also endometriosis, has been attracting attention.6 In particular, dydrogesterone does not inhibit ovulation, and does not affect basal body temperature (BBT). Thus, even while on treatment, ovulation may occur and pregnancy is possible. Although dydrogesterone is an old retro-progesterone preparation used for over a half century in Japan as well as in Europe, almost no clinical evidence in Japanese patients has been collected. Currently, new clinical data are requested mainly by gynecologists, for reverification of the efficacy, safety, and fertility improving the effect of dydrogesterone. For this reason, the clinical study was planned to re-evaluate the efficacy and safety of dydrogesterone in patients with dysmenorrhea.[3]
For the treatment of endometriosis, the dose of dydrogesterone was set 10 mg/day for 21 days (from day 5 to 25 of menstruation cycle) in the previous studies. The cases of pregnancy during or after dydrogesterone treatment were reported.13 Therefore, in this study, we set this dosage and duration not to avoid the possibility of pregnancy during treatment. There were no deaths or severe side effects in this study. Serious adverse events reported in this study were bacterial pharyngitis in one patient and postural orthostatic tachycardia syndrome in one patient. These events were serious because of hospitalization for treatment or prolongation of existing hospitalization, and both events were assessed as causally unrelated to the study drug. Both events were confirmed to have resolved or improved. The only adverse event leading to discontinuation was the serious adverse event of postural orthostatic tachycardia syndrome in one patient. As the adverse events of other progestins which are considered to be effective for dysmenorrhea, the occurrences of severe uterine bleeding by dienogest and breast cancer or thrombosis by medroxyprogesterone acetate have been reported. However, in the present study, such adverse events were not appeared.
Treatment of dydrogesterone during four menstrual cycles in women with primary or secondary dysmennorhea led to improvement with the efficacy maintained up to the 5th menstruation cycle. Progestogens have been considered as the alternatives, because they are inexpensive and may have a better side effect profile than other choices. While no published data exist regarding dydrogesterone for dysmenorrhea in Japanese women, this study proved that dydrogesterone is efficacious, safe, and clinically beneficial in patients with dysmenorrhea, thereby indicating that dydrogesterone can become a treatment option for the patients with dysmenorrhea.
References
[1] HUNAN CHUNKANG MEDICINE TECH - CN114957370, 2022, A
[2] Carp H. A systematic review of dydrogesterone for the treatment of threatened miscarriage. Gynecol Endocrinol. 2012 Dec;28(12):983-90.
[3] Taniguchi F, Ota I, Iba Y, Toda T, Tagashira Y, Ohata Y, Kurioka H, Endo Y, Sunada H, Noma H, Azuma Y, Harada T. The efficacy and safety of dydrogesterone for treatment of dysmenorrhea: An open-label multicenter clinical study. J Obstet Gynaecol Res. 2019 Jan;45(1):168-175.
- Related articles
- Related Qustion
Sodium stearate is the ester of sodium and stearic acid--basically a specialized form of a very hard soap that doesn't lather much.....
Mar 12,2025SurfactantNervonic acid is a long chain unsaturated fatty acid and a monounsaturated analog of lignoceric acid.....
Mar 13,2025SupplementsDydrogesterone
152-62-5You may like
- L-1-Phenylethylamine: Synthesis and Application
Mar 13, 2025
- Isothiazolinones: A Functional Biocides
Mar 12, 2025
- Guide to Polyethylene Terephthalate
Mar 12, 2025
- Dydrogesterone
-

- $1.00 / 1kg
- 2025-03-13
- CAS:152-62-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100kg
- Dydrogesterone
-

- $0.00 / 1g
- 2025-03-13
- CAS:152-62-5
- Min. Order: 1g
- Purity: 98.0%~102.0%(on dried basis),EP10.0
- Supply Ability: 10KG/month
- Dydrogesterone
-

- $99.00/ kg
- 2025-03-13
- CAS:152-62-5
- Min. Order: 0.00100000kg
- Purity: 99%
- Supply Ability: 5000