Disodium edetate dihydrate: Indication, Application, toxicity, Preparation
Apr 4,2023
Indication
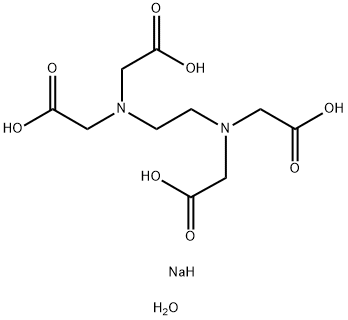
Disodium edetate dihydrate[1], also known as disodium ethylenediaminetetraacetic acid dihydrate (disodium EDTA dihydrate), is a chelating agent used in a variety of industries. It is a white, crystalline powder that is soluble in water. Disodium EDTA dihydrate is commonly used in the food industry as a preservative and to prevent discoloration caused by metal ions. It is also used in cosmetics and personal care products as a stabilizer and to improve the effectiveness of other ingredients. In addition, disodium EDTA dihydrate has medical applications, such as treating lead poisoning and calcium deposits in the blood vessels. It is also used in some medications as a stabilizer or to enhance absorption. However, it is important to note that disodium EDTA dihydrate can cause irritation and allergic reactions in some individuals, and should be handled with care.

Figure 1 Appearance of Disodium edetate dihydrate.
Application
In organic synthesis, Disodium edetate dihydrate is commonly used for the purification and preparation of metal-free compounds. One application of EDTA-Na2 in organic synthesis is its use as a metal scavenger. Metal ions can catalyze unwanted reactions or interfere with desired reactions in organic synthesis. Disodium edetate dihydrate can be added to reaction mixtures to sequester metal ions and prevent their interference. Disodium edetate dihydrate can also be used to remove metal impurities from organic compounds. Purification methods such as column chromatography and recrystallization are often insufficient to remove trace amounts of metal impurities. However, the addition of EDTA-Na2 to a solution containing metal impurities can effectively chelate the metal ions and facilitate their removal by extraction or filtration. Additionally, EDTA-Na2 can be used as a complexing agent in the synthesis of metal-organic frameworks (MOFs). MOFs are porous materials composed of metal ions held together by organic ligands. Disodium edetate dihydrate can be used as a chelating agent to form metal complexes, which can then be incorporated into MOFs. Overall, Disodium edetate dihydrate is an important tool in organic synthesis for the removal of metal impurities, the prevention of unwanted metal-catalyzed reactions, and the preparation of metal-free compounds.
Disodium ethylenediamine tetraacetic acid has 6 donor atoms, which can meet the metal ions with 6 coordination numbers[2]. When the electron pairs on the nitrogen atoms and the free electron pairs of the 4 carboxyl groups in disodium ethylenediamine tetraacetic acid coordinate with the metal ions, they can form a very stable complex 1, namely, iron, copper Polyvalent metal ions such as magnesium and calcium chelate to form stable water-soluble complexes. Their complexing effects can effectively prevent metal induced changes such as discoloration, oxidation, rancidity, turbidity, and vitamin C oxidation loss, thereby playing a role in color protection and oxidation resistance2. Therefore, they are often used as antioxidant, stabilizer, preservative, and coagulant in foods such as jam, canned vegetables, and preserved sweet potatoes[3].
Toxicity
Disodium edetate dihydrate cannot be decomposed in the human body and is excreted outside the body, which can cause iron deficiency in the human body and can also cause temporary blood pressure drop and renal dysfunction. Therefore, the accumulation of disodium ethylenediamine tetraacetic acid in the human body can damage the health of the body. Excessive or non compliant use can affect human health, not only leading to the loss of trace elements needed in the human body, causing symptoms such as vomiting, diarrhea, and even temporary renal dysfunction and lower blood pressure[4].
Preparation
Here is the synthesis of Disodium edetate dihydrate:
1. Ethylenediamine is reacted with chloroacetic acid to form the intermediate compound N-(2-carboxyethyl)ethylenediamine.
2. The N-(2-carboxyethyl)ethylenediamine is then reacted with sodium hydroxide to form the disodium salt.
3. Ethylenediaminetetraacetic acid (EDTA) is synthesized by reacting the disodium salt of N-(2-carboxyethyl)ethylenediamine with sodium cyanide and hydrogen peroxide.
4. The final step is to convert EDTA into its disodium salt form by reacting it with sodium hydroxide solution and then crystallizing it from aqueous solution to obtain Disodium edetate dihydrate.

Figure 2 Chemical reaction equation of Disodium edetate dihydrate.
References
[1] Yang Changzhi, Han Guangyuan, Jiang Bing, Wei Dongxu, Cheng Yang. Determination of residual disodium EDTA in Babao Congee cans by UPLC-MS-MS [J]. Food Science, 2015,36 (04): 208-212
[2] He Shukun. Study on the determination of EDTA in dried sweet potato by ultra high performance liquid chromatography [J]. Fujian Light Textile, 2009 (06): 31-33.
[3] Wei Dongxu, Hu Shen, Sun Ying, Hu Shaoxin, Han Guangyuan. Determination of disodium ethylenediaminetetraacetate in foods by high-performance liquid chromatography [J]. Journal of Inspection and Quarantine, 2013,23 (06): 50-53.
[4] Sun Yanmin, Pan Yuning, Lin Hui, Yan Chunrong, Xu Chunxiang. Research progress in the application of ethylenediamine tetraacetate in the food field [J]. Modern Food, 2019, (17): 1-5.
- Related articles
- Related Qustion
- Disodium Edetate Dihydrate: Pharmacokinetics, Mechanism of Action and Side Effects May 20, 2024
Disodium edetate dihydrate is a polyvalent ion chelator that can lower serum calcium levels and increase urinary excretion of metals, but side effects like renal irritation may occur.
- What is edetate disodium(EDTA)? Nov 19, 2019
EDTA is used to lower blood levels of calcium when they have become dangerously high. EDTA is also used to control heart rhythm disturbances caused by a heart medication called digitalis (digoxin, Lanoxin).
Acetaldehyde is a substance that is produced in the human body during metabolic processes, for example when the body breaks down alcohol. The taste of acetaldehyde is described as fresh with a fruity but sometimes musty odour.....
Apr 3,2023Organic ChemistryTosyl chloride is used in organic chemistry as a reagent for converting alcohols and amines into corresponding sulfonate esters or amides, respectively.....
Apr 4,2023APIDisodium edetate dihydrate
6381-92-6You may like
Disodium edetate dihydrate manufacturers
- Disodium edetate dihydrate
-

- $100.00 / 50kg
- 2024-12-22
- CAS:6381-92-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000Ton
- EDTA-2Na
-

- $0.00 / 1kg
- 2024-12-20
- CAS:6381-92-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT
- Disodium edetate dihydrate
-

- $120.00 / 1kg
- 2024-12-20
- CAS:6381-92-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton