Biphenyl: Chemical property and uses
Aug 13,2024
Introduction
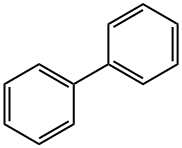
Biphenyl, an aromatic hydrocarbon, is used alone or with diphenyl ether as a heat-transfer fluid; the chemical formula is C6H5C6H5. It may be isolated from coal tar; in the United States, it is manufactured on a large scale by the thermal dehydrogenation of benzene.
Chemical property
Biphenyl is a colourless to yellow solid with a pleasant odour. It is soluble in alcohol, ether, benzene, methanol, carbon tetrachloride, carbon disulfide, and most organic solvents. It is very slightly soluble in water.
Biphenyl is one of the most thermally stable of all organic compounds. It is combustible at high temperatures, producing carbon dioxide and water when combustion is complete. Partial combustion produces carbon monoxide, smoke, soot, and low molecular weight hydrocarbons.
Detection method
Biphenyl is usually determined by chromatographic analysis of an extract prepared using an organic solvent. In the TLC method, biphenyl is located under UV radiation, extracted with methanol, and determined spectrophotometrically at 248 nm. If the silica gel layer is impregnated with the fluorescent indicator GF254, biphenyl can be detected by densitometry. LC analysis with UV detection at 254 nm and fluorimetric detection at 350 nm with excitation at 285 nm are good alternatives. Derivative infrared spectrometry has also been developed as an alternative procedure.
Uses
Its primary uses are producing heat-transfer fluids (for example, as an intermediate for polychlorinated biphenyls) and dye carriers for textile dyeing. Lesser uses are as a mould retardant in citrus fruit wrappers, in the formation of plastics, optical brighteners, and hydraulic fluids.
Biphenyl is non-reactive due to its lack of functional groups. The functional groups are the basis of its application. In labs, biphenyl is mainly used as a heat transfer agent or an eutectic mixture with diphenyl ether. This mixture is kept stable at 400 degrees. Biphenyl undergoes sulfonation, which, followed by base hydrolysis, produces p-hydroxyphenyl. This is a useful fungicide. These reactions undergo halogenation. Polychlorinated biphenyls, also known as PCB polychlorinated biphenyls, were once popular pesticides. Lithium biphenyl contains the radical anion. Many solvates of alkali metal salts of biphenyl anion have been characterized by X-ray crystallography. Lithium biphenyl offers an advantage relative to the related lithium naphthene.
- Related articles
- Related Qustion
- The unique characteristics of biphenyl Apr 15, 2022
Biphenyl is an organic compound with chemical formula of C12H10. It is a white crystalline powder, insoluble in water.
Paliperidone Palmitate stands as a beacon of progress in the treatment of psychiatric disorders, particularly schizophrenia and schizoaffective disorder.....
Dec 19,2024APIRoflumilast is a selective PDE4 inhibitor that inhibits pulmonary and systemic inflammation and rallies symptoms in airway diseases.....
Oct 21,2024API