產(chǎn)品屬性:
產(chǎn)品名稱 | 規(guī)格 | CAS號 | 型號 |
Marimastat | 10mM (in 1mL DMSO) 1mg 10mg 50mg | 154039-60-8 | EY-Y0164366 |
Cas No.154039-60-8
別名
化學(xué)名 (2R,3S)-N-[(2S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl]-N',3-dihydroxy-2-(2-methylpropyl)butanediamide
分子式 C15H29N3O5
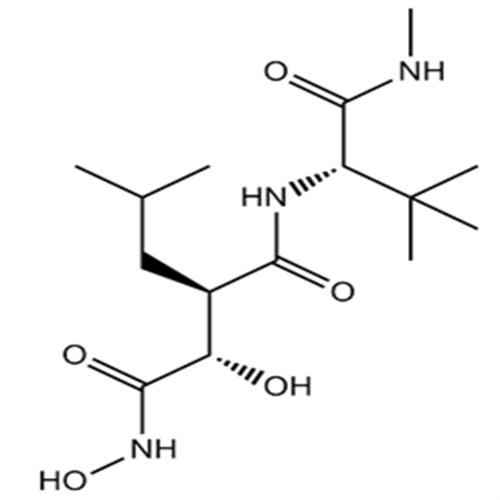
分子量 331.41
溶解度 ≥ 80.1 mg/mL in DMSO, ≥ 20.43 mg/mL in EtOH, ≥ 2.8 mg/mL in H2O with ultrasonic and warming
儲存條件 Store at -20°C
General tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.
Shipping Condition Evaluation sample solution : ship with blue ice
All other available size: ship with RT , or blue ice upon request
產(chǎn)品描述:
Marimastat is a broad range inhibitor of Matrix metalloproteinases with IC50 values of 5, 6, 13, 3 and 9 nM for MMP-1, MMP-2, MMP-7 , MMP-9 and MMP-14 [1].
Marimastat is an orally bioavailable inhibitor with an absolute bioavailability of 20%–50% in preclinical studies, which can bind covalently to the zinc atom of the MMP-active site by a collagen-mimicking hydroxamate structure [1].
A reduction in the size and number of metastatic foci in treated compared with the control animals was demonstrated by experimental metastases models against lung and breast cancer. The studies were carried out at doses of 100–500 mg/kg per day, and the agent induced gastrointestinal toxicity and weight loss, as well as hemorrhage, fibrosis, inflammation, and necrosis at particular ankle and knee tissues. Single oral doses of up to 800 mg were well tolerated and did not lead to obvious toxicity. Peak plasma concentrations can be detected within 1.5–3 hours after oral administration, and the elimination half-life was estimated as a range of 8–10 hours. No plasma accumulation was detected after an oral doses of 50–200 mg in continuous administration twice a day for 6 consecutive days [2,3].
Pharmacological studies demonstrated that marimastat is well absorbed from the gastrointestinal tract and exhibits a linear pharmacokinetic behavior. The minimum plasma concentration was found after exceeding 10 mg doses twice a day, which were sixfold greater than the required for inhibition of MMP in vitro. Complain to the patients treated with gemcitabine, the most effective chemotherapeutic agent against the nonmetastatic pancreatic cancer, the patients who received high doses of marimastat had a 1-year survival rates. It is encouraging that the patients with unresectable gastric cancer who were treated with marimastat show a modest increase in survival [1,2].
References:
1.Hidalgo M, Eckhardt SG, Development of matrix metalloproteinase inhibitors in cancer therapy. JOURNAL OF THE NATIONAL CANCER INSTITUTE. 2001, 93(3):178-193.
2.Coussens LM, Fingleton B , Matrisian LM , Cancer therapy - Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. SCIENCE, 295 (5564): 2387-2392.
3.Van Wijngaarden J , Snoeks TJA , van Beek E, et al . An in vitro model that can distinguish between effects on angiogenesis and on established vasculature: Actions of TNP-470, marimastat and the tubulin-binding agent Ang-510. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS, 2000, 391 (2): 1161-1165.
關(guān)鍵字: 154039-60-8;C15H29N3O5;Marimastat;
上海一研生物科技有限公司Shanghai yiyan bio-technology Co. Ltd.主要從事免疫學(xué)、分子生物學(xué)和常規(guī)生化試劑等為一體的科研產(chǎn)品銷售企業(yè),公司自成立以來,秉承""全心全意服務(wù)于科研工作者""的企業(yè)理念���,立足生物科技領(lǐng)域�,運(yùn)用生物技術(shù)和科研試劑�����,發(fā)展現(xiàn)代生物科技�����,為各類大中小醫(yī)院及其它醫(yī)療機(jī)構(gòu)���、高等院校、科研院所��、企事業(yè)單位提供優(yōu)質(zhì)的產(chǎn)品�,服務(wù)生物科技領(lǐng)域的科學(xué)研究人員。
公司具有對普通貨物���、冷藏及冷凍倉庫的存儲����、包裝及運(yùn)輸能力���。
公司將始終堅持信譽(yù)立業(yè)��、以人為本���、質(zhì)量保證���、誠信服務(wù)的宗旨,不斷拼搏�,開拓進(jìn)取,與各界朋友攜手共創(chuàng)美好未來����。