| 89.6% |
With phenylboronic acid; In toluene;Reflux; |
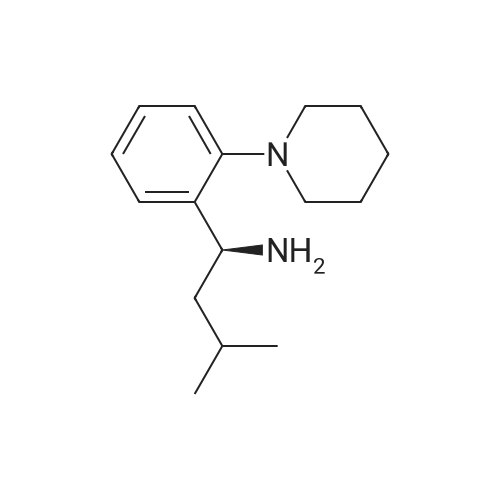
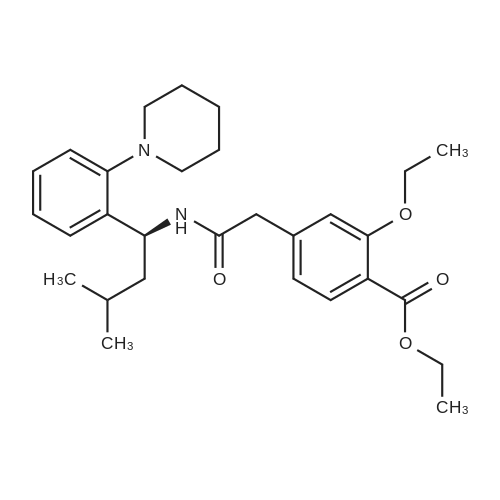
Example 8Preparation of Ethyl (S)-2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoateIn a round bottom flask fitted with a Dean Stark condenser, 3-ethoxy-4-ethoxycarbonyl phenyl acetic acid (10.26 g, 0.0426 moles) was dissolved in toluene (100 ml) followed by slow addition of phenylboronic acid (0.494 g, 0.0040 moles) and (S)-3-methyl-1-(2-piperidinophenyl)-1-butylamine (10 g, 0.0406 moles). The reaction mixture was refluxed for 16-18 hours. The reaction mixture was cooled at room temperature followed by filtration. The toluene layer was washed with water and 1% sodium bicarbonate solution. This was followed by complete distillation of toluene. Hexane (50 ml) was added to the resulting residue in order to precipitate the solid and then stirred for 1 hour. The resulting solid was filtered and washed with hexane (10 ml). The wet material was further dried at 50-55 C. under vacuum for 4-6 hours to produce ethyl (S)-2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoate (Yield: 89.6%; HPLC purity: 99.46%; and Chiral purity: 99.98%). |
| 87.2% |
With tetramethylorthosilicate; In toluene; for 11h;Inert atmosphere; Reflux; |
Under nitrogen, compound 370.3g (0.285mol, 1eq), 4- carboxy-methyl-2-ethoxy ethyl benzoate 72.0g (0.285mol ,, 1eq), tetramethoxysilane 86.8g (152.22,0.57 mol, 2eq) and 285g of toluene, heated to reflux for 11 hours, HPLC control the starting material the reaction was complete, cooled, concentrated under reduced pressure, from ethanol / MTBE to give white crystalline solid 119.4g, HPLC98.8%, yield 87.2%, 99.5% ee. |
| 73.3% |
With boric acid; In toluene; for 16 - 18h;Heating / reflux;Product distribution / selectivity; |
EXAMPLE 1 Preparation of Ethyl (S)-2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoate In a round bottom flask fitted with a dean stark condenser, (S)-3-methyl-1-(2-piperidinophenyl)-1-butylamine (10 g, 0.0406 mol) was dissolved in toluene (100 ml), followed by the addition of 3-ethoxy-4-ethoxycarbonyl phenyl acetic acid (10.26 g, 0.0407 mol) and boric acid (0.26 g, 0.0042 mol). The reaction mixture was refluxed for 16-18 hours. The resulting mass was then cooled to 25-30 C. followed by filtration. The filtrate was washed with water and 1.0% sodium bicarbonate solution followed by complete distillation of toluene and the resulting residue was stirred with hexane (50 ml) for 1 hour. The precipitated solid was filtered and washed with hexane (10 ml). The wet product was dried at 50-55 C. under vacuum for 4-6 hours to produce Ethyl (S)-2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)-aminocarbonyl methyl]-benzoate (Yield=73.3%; HPLC Purity: 99.50%). |
|
With triethylamine; triphenylphosphine; In tetrachloromethane; acetonitrile; |
EXAMPLE 1 Ethyl (S)-2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)-aminocarbonylmethyl]-benzoate 0.48 g (1.91 mMol) of 3-ethoxy-4-ethoxycarbonylphenylacetic acid, 0.60 g (2.29 mMol) of triphenylphosphine, 0.80 ml (5.73 mMol) of triethylamine and 0.18 ml (1.91 mMol) of carbon tetrachloride are added successively to a solution of 0.47 g (1.91 mMol) of (S)-3-methyl-1-(2-piperidino-phenyl)-1-butylamine (ee=98.5%) in 5 ml of anhydrous acetonitrile and the resulting mixture is stirred for 20 hours at ambient temperature. It is then evaporated down in vacuo and distributed between ethyl acetate and water. The organic extract is dried and filtered and evaporated down in vauco. The evaporation residue is purified by column chromatography on silica gel (toluene/ethyl acetate=10/1). Yield: 0.71 g (77.3% of theory), Melting point: 110-112 C. Calculated: C 72.47; H 8.39; N 5.83. Found: C 72.29; H 8.42; N 5.80. |
|
With dicyclohexyl-carbodiimide; In toluene; at 25 - 40℃; |
EXAMPLE 5; PREPARATION OF REPAGLINIDE; 3-Ethoxy-4-(ethoxycarbonyl)benzene acetic acid (2.25 g, 0.0089 moles) was added to a solution of (alphaS)-alpha-(2-methylpropyl)-2-(1-piperidinyl)benzenemethanamine (2g, .008 moles) in toluene (20 ml). Then N,N-dicyclohexylcarbodiimide (1.95 g, 0.0094 moles) was added and the reaction mixture was stirred till completion of the reaction at 25-40C. The solid was filtered under suction and toluene filtrate was distilled under reduced pressure. The product was crystallized from ethanol / water and dried to yield ethyl 2-ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzoate (3 g). 1N aqueous sodium hydroxide solution (8.0 ml) in ethanol (30 ml) was added to ethyl-2-ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzoate (3 g) at 60-65C and stirred. After completion of the reaction the reaction mixture was cooled, pH was adjusted to 5 with 1N aqueous hydrochloric acid and product was filtered and dried at reduced pressure. The dried product was recrystallised from aqueous ethanol to give repaglinide. Yield: 2.27 g |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping