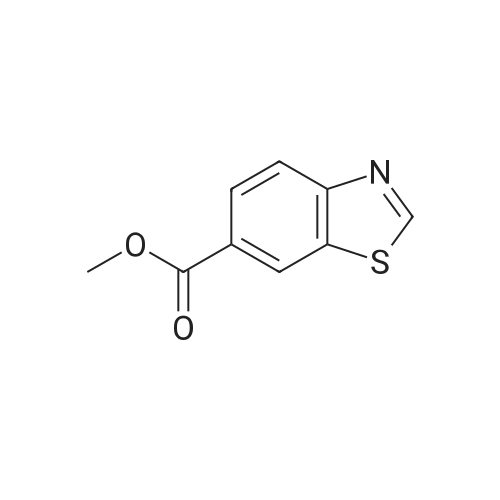
|
|
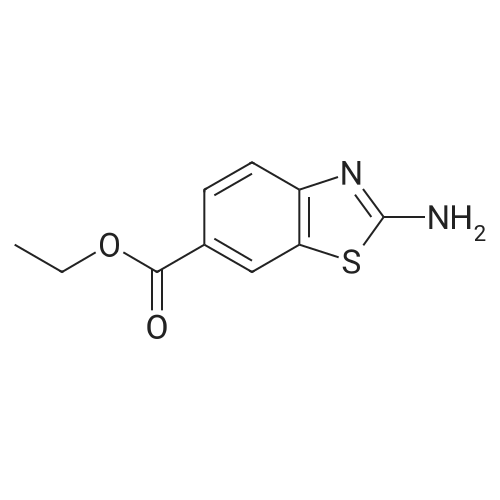
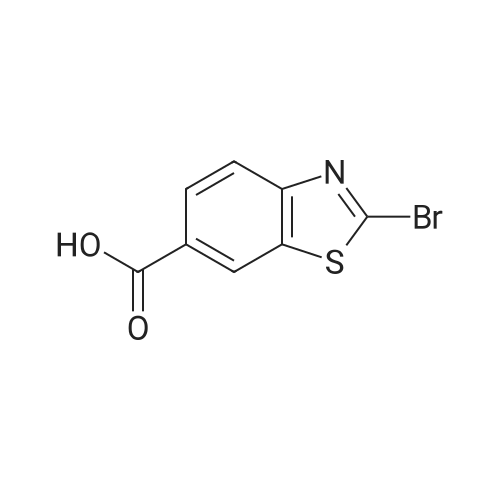
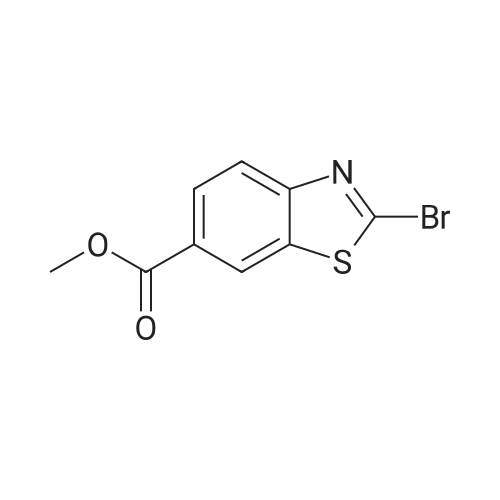
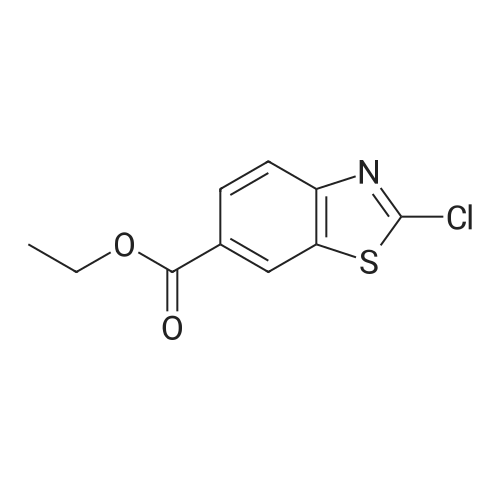
(195-4) Ethyl 2-bromo-1,3-benzothiazole-6-carboxylate was obtained from the compound of Example 195-3 in a similar manner to Example 125-2. 1H NMR (CDCl3, 400MHz) delta 9.71 (brs, 1H), 9.16 (s, 1H), 8.56 (d, 1H, J=1.2Hz), 8.24 (d, 1H, J=8.5Hz), 8.09 (dd, 1H, J=8.5, 1.2Hz), 7.19 (m, 1H), 6.95 (m, 1H), 6.39 (m, 1H). |
|
With tert.-butylnitrite; copper(ll) bromide; In acetonitrile; at 0 - 20℃; for 2.16667h;Inert atmosphere; |
EXAMPLE 1; 2-[l-(3,4,5-Tribromobenzyl)-lH-L2,3-triazol-4-yl]-L3-benzothiazole-6-carboxylic acid Step 1: Ethyl 2-bromo-L3-benzothiazole-6-carboxylateA suspension Of CuBr2 (1.20 g, 5.40 mmol) in anhydrous acetonitrile (15 niL) was purged with nitrogen. The mixture was cooled in an ice-bath and treated with t- BuONO (696 mg, 6.75 mmol). After further stirring for 10 min at about 0 to 5 0C, the mixture was reacted with 2-amino-l,3-benzothiazole-6-carboxylate (1.0 g, 4.5 mmol). The cooling bath was removed and the reaction mixture was stirred at room temperature for 2 h. The mixture was then diluted with water (30 mL) and extracted with ether (100 mL x 2). The combined organic phase was filtered to removed copper salts, then washed with water (20 mL) and brine (20 mL), dried over Na2SO4 and concentrated to afford the title compound.1H NMR (CDCl3, 400 MHz): delta 8.54 (d, IH), 8.16 (dd, IH), 8.02 (d, IH), 4.42 (q, 2H), 1.42 (t, 3H). |
|
With tert.-butylnitrite; copper(ll) bromide; In acetonitrile; at 65℃; for 2h; |
STEP A: Ethyl 2-aminobenzothiazole-6-carboxylate (8.91 g) and CuBr2 (13.43 g) were combined in acetonitrile (300 mL). To the resulting deep green solution was added tert-butylnitrite (7.14 mL). The resulting mixture was heated to ~65 C for two hours, then concentrated to ~50 mL. The resulting concentrate was diluted with water (250 mL) and extracted with EtOAc (2*250 mL). The resulting yellow solution was concentrated to yield ethyl 2-bromobenzo[d]thiazole-6-carboxylate as a yellow solid. 1H NMR delta 8.55 (s, 1H), 8.16 (dd, 1H, J=8.6, 1.7 Hz), 8.03 (d, 1H, J=8.3 Hz), 4.43 (q, 2H, J=7.1 Hz), 1.43 (t, 3H, J=7.1 Hz). MS: 286.0 (M+H). |
|
With tert.-butylnitrite; copper(ll) bromide; In acetonitrile; at 20℃; for 14h;Cooling with ice; |
To the solution of ethyl 2-aminobenzo[d]thiazole-6-carboxylate (8.0 g, 36.0 mmol) and CuBr2 (16.07 g, 72.0 mmol) in acetonitrile (200 mL), tert-butyl nitrite (7.42 mL, 72.0 mmol) was added on ice bath. The reaction mixture was stirred at room temperature for 14 h. The solvent was removed under reduced pressure and to the residue ethyl acetate (200 mL) and NH4Cl solution (200 mL) were added. The organic phase was washed with brine (100 mL) and NH4Cl solution (100 mL), dried over Na2SO4, filtered and the solvent evaporated under reduced pressure. Yield: 8.0 g (78%); pale brown solid. (0862) 1H NMR (400 MHz, DMSO-d6): d 1.36 (t, J = 7.1 Hz, 3H), 4.37 (q, J = 7.1 Hz, 2H), 8.10 (s, 2H), 8.82 (s, 1H) ppm. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping