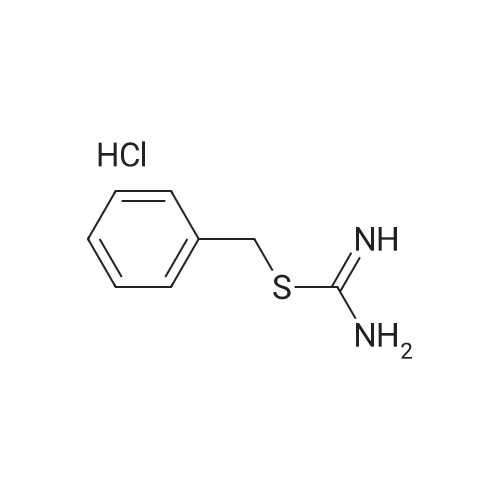
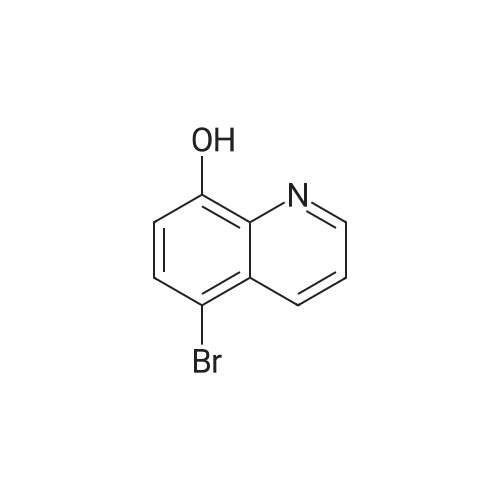
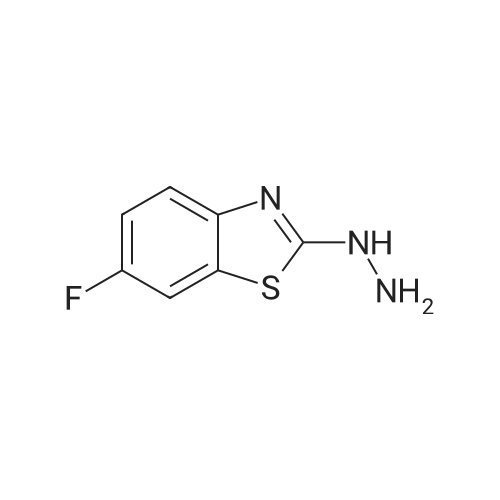
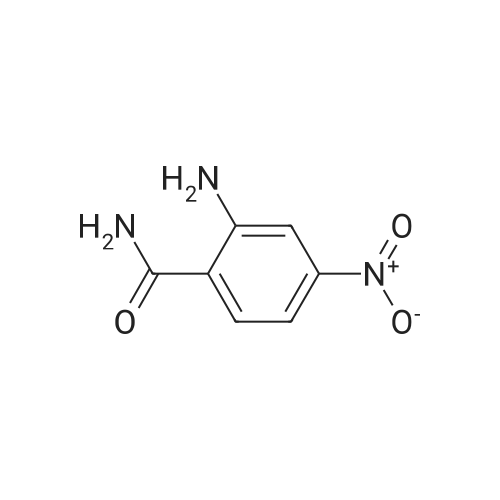
Robenidine derivatives as potential antischistosomal drug candidates
Christian N. Lotz
;
Alina Krollenbrock
;
Lea Imhof
, et al.
Int J Parasitol-Drug,2024,25,100546.
DOI:
10.1016/j.ijpddr.2024.100546
More
Abstract: Schistosomiasis caused by Schistosoma spp. is a disease that causes a considerable health burden to millions of people worldwide. The limited availability of effective drugs on the market and the increased risk of resistance development due to extensive usage, highlight the urgent need for new antischistosomal drugs. Recent studies have shown that robenidine derivatives, containing an aminoguanidine core, exhibit promising activities against Plasmodium falciparum, motivating further investigation into their efficacy against Schistosoma mansoni, due to their similar habitat and the resulting related cellular mechanisms like the heme detoxification pathway. The conducted phenotypic screening of robenidine and 80 derivatives against newly transformed schistosomula and adult Schistosoma mansoni yielded 11 candidates with low EC50 values for newly transformed schistosomula (1.12–4.63 μM) and adults (2.78–9.47 μM). The structure-activity relationship revealed that electron-withdrawing groups at the phenyl moiety, as well as the presence of methyl groups adjacent to the guanidine moiety, enhanced the activity of derivatives against both stages of Schistosoma mansoni. The two compounds 2,2′-Bis[(3-cyano-4-fluorophenyl)methylene] carbonimidic Dihydrazide Hydrochloride (1) and 2,2′-Bis[(4-difluoromethoxyphenyl) ethylidene] carbonimidic Dihydrazide Hydrochloride (19), were selected for an in vivo study in Schistosoma mansoni-infected mice based on their potency, cytotoxicity, pharmacokinetic-, and physicochemical properties, but failed to reduce the worm burden significantly (worm burden reduction <20%). Thus, robenidine derivatives require further refinements to obtain higher antischistosomal specificity and in vivo activity.
Keywords:
Robenidine derivative ;
Aminoguanidine ;
Schistosoma mansoni ;
Drug discovery ;
Structure-activity relationship
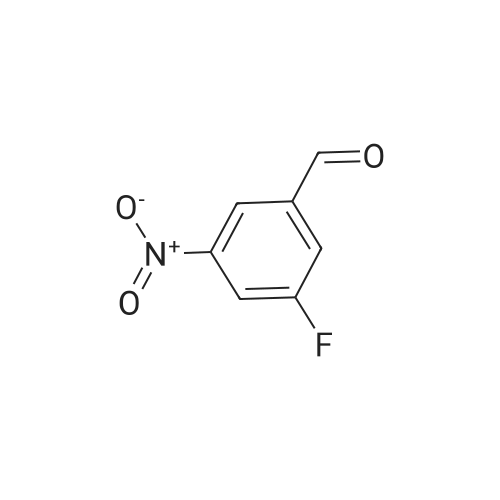
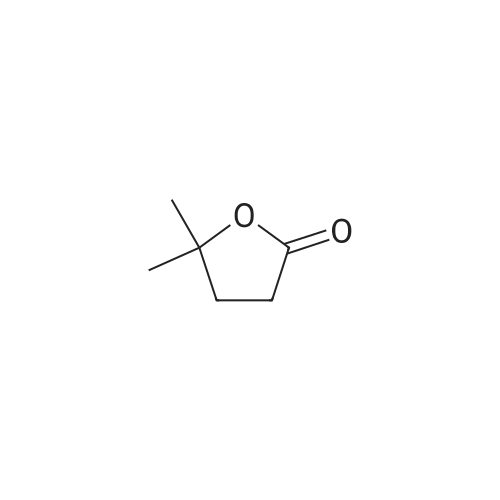
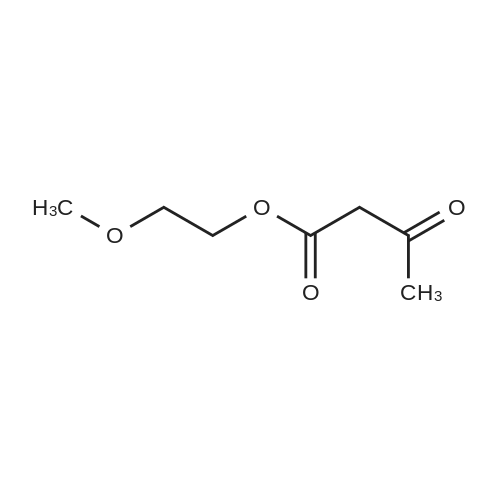
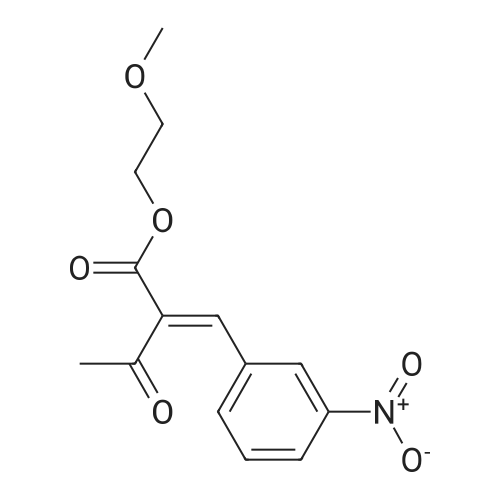
Purchased from AmBeed:
101048-76-4 ;
90-98-2 ;
36062-19-8 ;
99-61-6 ;
1261759-41-4 ;
218301-22-5 ;
67-36-7 ;
35714-48-8

Are β-Lactones Involved in Carbon-Based Olefination Reactions?
Jan Nowak
;
Micha? Tryniszewski
;
Micha? Barbasiewicz
Synlett,2024,35(10):1190-1194.
DOI:
10.1055/a-2268-4386
More
Abstract: Heteroatom-based olefinating reagents (e.g., organic phosphonates, sulfonates, etc.) are used to transform carbonyl compounds into alkenes, and their mechanism of action involves aldol-type addition, cyclization, and fragmentation of four-membered ring intermediates. We have developed an analogous process using ethyl 1,1,1,3,3,3-hexafluoroisopropyl methylmalonate, which converts electrophilic aryl aldehydes into α-methylcinnamates in up to 70% yield. The reaction plausibly proceeds through the formation of β-lactone that spontaneously decarboxylates under the reaction conditions. The results shed light on the Knoevenagel–Doebner olefination, for which decarboxylative anti-fragmentation of aldol-type adducts is usually considered.
Keywords:
olefination ;
carbonyl compounds ;
reaction mechanism ;
lactones ;
malonates ;
Knoevenagel ;
Doebner reaction
Purchased from AmBeed:
587-04-2 ;
609-08-5 ;
29166-72-1 ;
99-61-6 ;
555-16-8 ;
104-88-1 ;
6674-22-2
Arylnitro monocarbonyl curcumin analogues: Synthesis and in vitro antitubercular evaluation
du Preez, Charne
;
Legoabe, Lesetja J.
;
Jordaan, Audrey
, et al.
Chem. Biol. Drug Des.,2023,101(3):717-726.
DOI:
10.1111/cbdd.14174
PubMed ID:
36350112
More
Abstract: Curcumin is a natural product that has been reported to exhibit myriad pharmacol. properties, one of which is antitubercular activity. It demonstrates antitubercular activity by directly inhibiting Mycobacterium tuberculosis (M.tb) and also enhances immune responses that ultimately lead to the elimination of M.tb by macrophages. This natural product is, however, unstable, and several analogs, noticeably monocarbonyl analogs, have been synthesized to overcome this challenge. Curcumin and its monocarbonyl analogs reported so far exhibit moderate antitubercular activity in the range of 7 to 16 μM. Herein, we report a straightforward synthesis of novel monocarbonyl curcumin analogs, their antitubercular activity, and the structure-activity relationship. The hit compound from this study, 3a, exhibits potent MIC90 values in the range of 0.2 to 0.9 μM in both ADC and CAS media.
Keywords:
analogues ;
aryl nitro ;
curcumin ;
synthesis ;
tuberculosis
Purchased from AmBeed:
552-89-6 ;
458-37-7 ;
698-63-5 ;
99-61-6 ;
30626-03-0 ;
30625-98-0 ;
1675220-86-6 ;
30625-99-1


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping