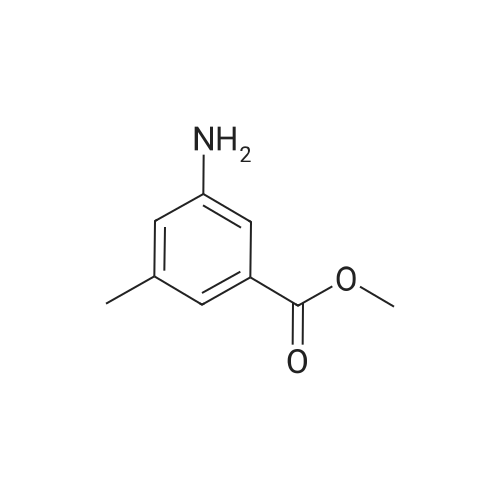
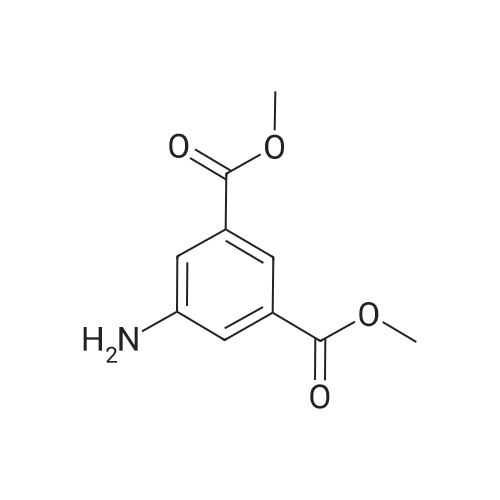
| 44% |
Stage #1: With hydrogenchloride; sodium nitrite In water at -5℃; for 0.25 h;
Stage #2: With fluoroboric acid In water at 0℃; for 0.5 h;
Stage #3: at 110℃; |
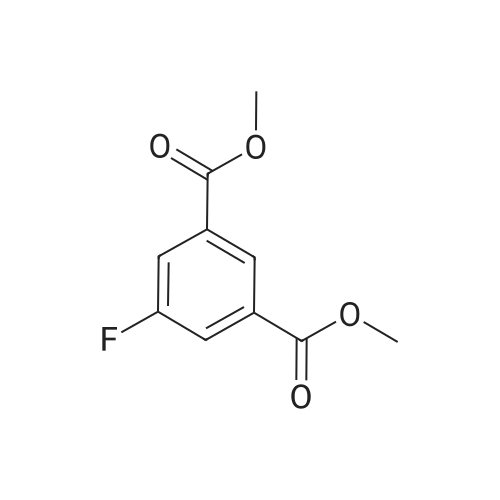
Concentrated [HC1] (30 ml) was added to a cooled [(-5°C)] suspension of dimethyl 5-amino isophthalate (20 g, 95.6 mmol) in water (75 ml), followed by portionwise addition of [NAN02] (7.5 g, 109 mmol). The reaction mixture was then stirred for 15 min. , after which [HBF4 (18] ml, 100 mmol, 48percent aqueous solution) was added. The resulting mixture was stirred at [0°C] for 30 min. and the precipitate formed was collected by filtration and washed with cold methanol (60 ml) and ether (60 ml). The residue was then decomposed by heating in an oil bath [(~110°C).] The cooled mixture was then diluted with ether, concentrated onto silica gel and purified by flash chromatography with 5percent ethyl acetate hexane as eluant giving 9.0 g (44percent) of product as a white fluffy [SOLID. LH NE (CDC13), 6] [(PPM)] : 8.57 (s, 1H), 7.95 (d, 2H), 3.97 (s, 6H). A suspension of 5-fluoro-isophthalic acid dimethyl ester (1.7 g, 8. 0 mmol) in methanol (41 ml) was treated with 1.0 N sodium hydroxide (7.2 ml, 7.2 mmol). The reaction was left stirring overnight at room temperature. After the solution was concentrated, the residue was dissolved in water and transferred to a separatory funnel. The aqueous layer was washed with dichloromethane (3 times) and then acidified with 1.0 N [HC1] to pH 2. Ethyl acetate was used to extract the precipitate, which was then washed with brine and dried over anhydrous sodium sulphate. After removal of solvent in vacuo, a total of 1.3 g (83percent) of 5-fluoro-isophthalic acid monomethyl ester was isolated as a white [SOLID. LH] NMR (DMSO), [5] (ppm): 8.31 (t, 1H), 7.96 (m, 2H), 3.91 (s, 3H). Triethylamine (2.2 ml, 16.0 mmol) and isobutyl [CHLOROFORMATE] (1.0 ml, 8. 0 mmol) were added to an ice-cooled solution [OF 5-FLUORO-ISOPHTHALIC] acid monomethyl ester (1.3 g, 6.7 mmol) in dichloromethane (20 ml) and then warmed to room temperature. After stirring for 2 h, the reaction mixture was filtered and concentrated. The residue was re-dissolved tetrahydrofuran (10 ml) and then sodium borohydride [(1.] 1 g, 29.02 mmol) in water (3ml) was added drop-wise. After 1 h, the reaction was quenched with methanol and then diluted with ethyl acetate, washed with water and brine, dried over anhydrous sodium sulfate, filtered and concentrated. Flash column chromatography on silica gel using 30percent ethyl acetate in hexanes afforded 667 mg (54percent) of 3-fluoro-5-hydroxymethyl-benzoic acid methyl ester as a colorless [OIL. 1H] NMR [(CDC13),] [8] (ppm): 7.82 (s, 1H), 7.63 (d, 1H), 7.32 (d, 1H), 4.76 (s, 2H), 3.93 (s, 3H). Ethanol (2 ml) was added to round bottom flask containing 3-fluoro-5-hydroxymethyl- benzoic acid methyl ester (667 mg, 3.6 mmol) and palladium (10 wt. percent on activated carbon, 300 mg) under argon. The flask was evacuated using a water aspirator and then filled with hydrogen from a balloon. After stirring for 2 h, the palladium on carbon was removed by filtration through celite. The filtrate was then concentrated to afford 520 mg (87percent) of 3-fluoro-5-methyl-benzoic acid methyl [ESTER. LH] NMR [(CDC13),] 8 (ppm): 7.65 (s, 1H), 7.51 (d, 1H), 7.08 (d, 1H), 3.91 (s, 3H), 2.40 (s, 3H). 0.5 N Lithium hydroxide (7.4 ml, 3.7 mmol) was added to a solution 3-fluoro-5-methyl- benzoic acid methyl ester (520 mg, 3.1 mmol) in tetrahydrofuran (7.4 ml). The reaction was stirred at [75 C] for 2 h and then the solvent was removed in vacuo. The residue was dissolved in a small amount of water and then acidified (pH about 2) by the addition of 10percent [HC1] (aq. ). Following extraction of the aqueous layer with ethyl acetate, the organic layer was then washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to afford 469 mg (98percent) [OF 3-FLUORO-5-METHYL-BENZOIC] acid as a white solid. 1H NMR (DMSO), d (ppm): 7.62 (s, 1H), 7.45 (d, 1H), 7.32 (d, 1H), 2.38 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping