| 93% |
With sodium hydroxide; In water; isopropyl alcohol; at 0℃; for 4h;Product distribution / selectivity; |
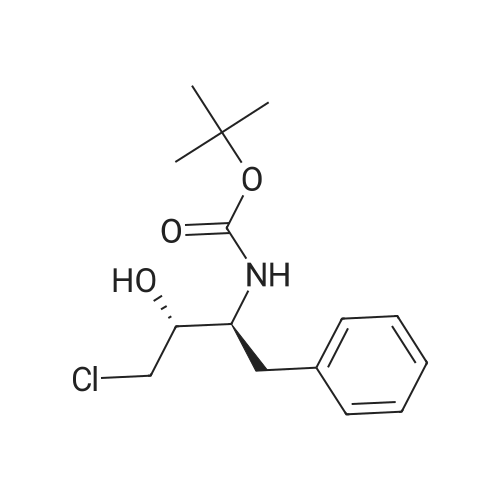
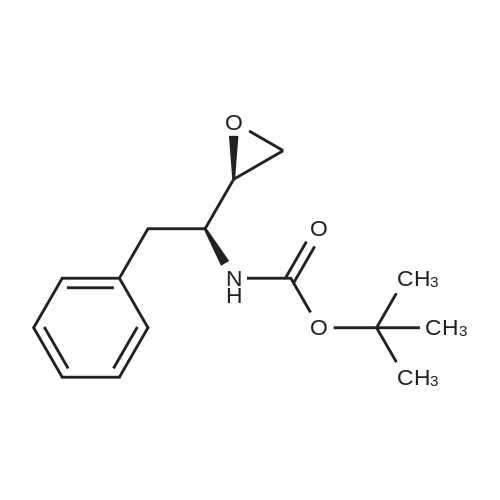
To (2R,3S)-3-tert-butoxycarbonylamino-1-chloro-2-hydroxy-4-phenylbutane (45.1 g) obtained in Example 3 were added isopropanol (120 ml) and water (45 ml), and the mixture was cooled to 0C. 29%. Aqueous sodium hydroxide solution was added, and the mixture was stirred for 4 hours. Aqueous citric acid solution (a mixed solution of citric acid (6.73 g) and water (14 ml)) was added to the reaction mixture, and acetone (35 ml) and water (59.5 ml) were further added. A seed crystal of (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-4-phenylbutane was added, and this mixture was stirred for 1 hour. Water (200 ml) was added dropwise to the mixture over 1 hour, and the mixture was stirred overnight. The slurry solution was filtered, and the crystals were washed twice with aqueous acetone solution (a mixed solution of acetone (50 ml) and water (350 ml)). Wet crystals were dried under reduced pressure at room temperature to give (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-4-phenylbutane as white crystals (37.1 g, 100 wt%, yield 93%). As a result of HPLC analysis, it was found that the peak area ratio of the compound was 99.9%, and the diastereomer ((2S,3S)-form) was not detected. |
| 83% |
With sodium hydroxide; In water; isopropyl alcohol; at 0℃; for 8.5h;Product distribution / selectivity; |
The organic layer (26.5 g) obtained in Comparative Example 2, step (2'g), was concentrated under reduced pressure, isopropanol (2 ml) was added to the residue, and the mixture was concentrated again to dryness. Isopropanol (21.8 ml) and water (3.0 ml) were added to the residue and the mixture was cooled to 0C. Then, 6 M aqueous sodium hydroxide solution (2.7 ml) and water (1.2 ml) were added to the solution, and the mixture was reacted for 8.5 hours. An aqueous solution (36.6 ml) of citric acid (351 mg) was added to the reaction mixture, and the mixture was cooled from 0C to -10C over 3 hours. A seed crystal of (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-4-phenylbutane was added, and the mixture was stirred at -10C for 3 days and filtered. The obtained crystals were dried under reduced pressure to give adhesive orange crystals (2.47 g). As a result of HPLC analysis, it was found that the content of the object compound, (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-4-phenylbutane, was 2.07 g, 83.8 wt%, and the yield was 83%. In addition, the crystals contained 0.071 g of a diastereomer ((2S,3S)-form)), and the diastereomer ratio (2R,3S)/(2S,3S) was 96.7/3.3. Moreover, the peak area ratio of other byproduct was 10% relative to the object compound, (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-4-phenylbutane. |
| 81.4 mg (93.5%) |
With potassium carbonate; citric acid; In methanol; water; ethyl acetate; |
Example 6 Production of (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-4-phenylbutane (2R,3S)-3-tert-butoxycarbonylamino-1-chloro-2-hydroxy-4-phenylbutane (100 mg) and potassium carbonate (91.5 mg) were added to methanol (2.0 ml) for agitation at ambient temperature for 4 hours. Aqueous 10% citric acid solution (0.204 ml) and water (0.408 ml) were added to the resulting mixture, from which the solvent was evaporated under reduced pressure. To the residue were added water (1 ml) and ethyl acetate (1 ml) for extraction; the organic phase was concentrated under reduced pressure, to afford (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-4-phenylbutane {(2R,3S) yield: 81.4 mg (93.5%)}. 1H-NMR (CDCl3, 300 MHz) delta ppm: 1.38 (s, 9H), 2.59 (bs, 1H), 2.69 (t. J=4.4 Hz, 1H), 2.83-3.04 (m, 3H), 4.12 (bs, 1H), 4.48 (bs, 1H), 7.17-7.37 (m, 5H) Mass spectrum m/e: 286 (M+Na+) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping