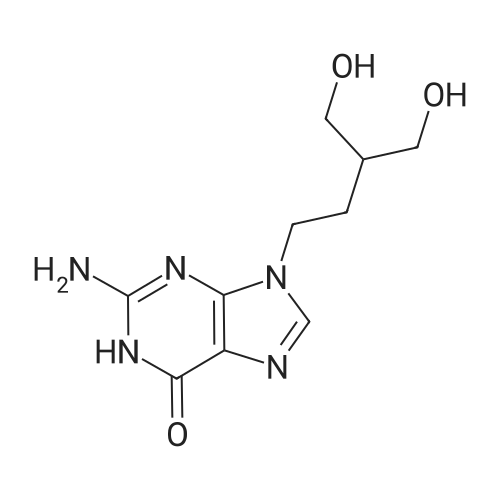
| 90.9% |
With water; ammonium formate;palladium on charcoal; In ethyl acetate; for 2h;Product distribution / selectivity; |
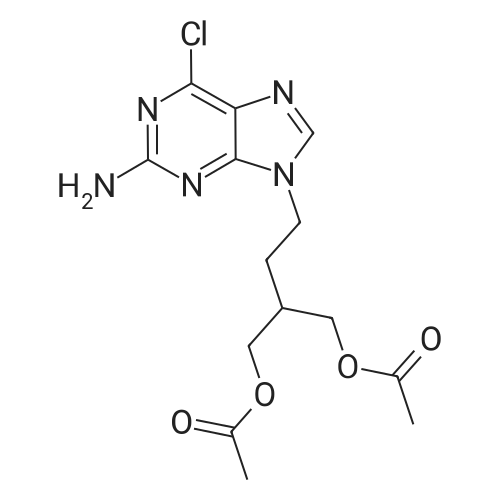
Into a jacketed reactor equipment with a mechanical stirrer, a reflux condenser and a thermocouple, under an inert atmosphere (N2), was added wet 10 % PD/C (4 g, 50 % HA0), EtOAc (220 ml), C1-FMC (20 g; 56.1 mmol) and ammonium formate (4.37 G ; 67.28 mmol ; 20 % excess). The reaction was completed after 2 hours, as all the CL-FMC was consumed. The reaction mixture was filtered at 50C and the filtrate was evaporated to dryness, leaving 16.4 g of solid (90.9 % of the 18 g expected). |
| 90% |
With ammonium formate;palladium; In methanol; water; |
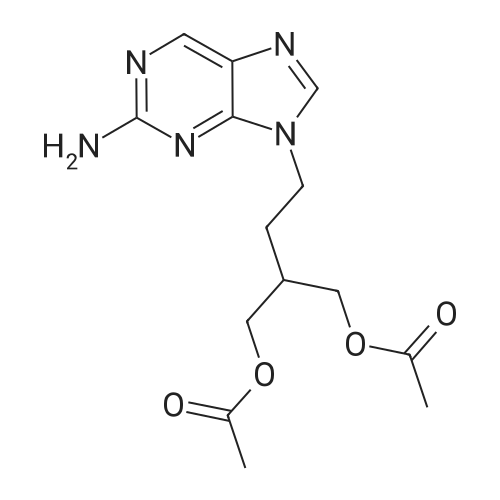
Example 2 9-(4-Acetoxy-3-acetoxymethylbut-1-yl)-2-aminopurine A suspension of 9-(4-acetoxy-3-acetoxymethylbut-1-yl)-2-amino-6-chloropurine (0.36g, 1.0mmol) and 10% palladium-on-charcoal (30mg) in methanol containing ammonium formate (400mM , 10ml) was heated under reflux for 30 minutes. The mixture was allowed to cool, filtered and the solvent removed. The residue was taken up in water and the solution extracted twice with chloroform. The organic layers were combined, dried (magnesium sulphate) and the solvent removed to afford 9-(4-acetoxy-3-acetoxymethylbut-1-yl)-2-aminopurine (0.29g, 90%). Recrystallisation from ethyl acetate-hexane gave white shiny plates (0.25g, 78%) m.p. 102-104C; λmax (MeOH) 222 (27,500), 244 (4,890), and 309 (7,160)nm; νmax (KBr) 3340, 3170, 1745, 1730, 1660, 1615 and 1580cmmin1; δH(CDCl3) 1.90-2.05 (3H, m, 2'-H and 3'-H), 2.07 (6H, s, 2 x CH3), 4.15 (4H, d, J 5.2 Hz, 2x4'-H), 4.21 (2H, t, J 7.2Hz, 1'-H), 5.16 (2H, br s, 2-NH2), 7.79 (1H, s, 8-H), and 8.70 (1H, s, 6-H); (Found: C, 52.10; H, 6.00; N, 21.49%. C14H19N5O4 requires C, 52.33; H, 5.96; N, 21.79%). |
| 90% |
With ammonium formate;palladium 10% on activated carbon; In methanol; for 2h;Heating / reflux; |
A mixture of 9-[4-acetoxy-3-(acetoxymethyl)butyl]-2-amino-6-chloro-purine (28.1 mmoles, 10 g), prepared according to Example 3 or 4, 10% palladium on charcoal (0.833 g) and ammonium formate (4 eq/mole, 7.08 g) in methanol (270 ml) is refluxed for 2 h under stirring. The mixture is cooled to room temperature and filtered and the filtrate is evaporated under reduced pressure to give a thick, colourless oil. The residue is then taken up into water (150 ml) and extracted with chloroform (2x100 ml). The combined organic phases are dried over anhydrous sodium sulfate and evaporated under reduced pressure. The crude is purified by crystallization from ethyl acetate/hexane to afford 9-[4-acetoxy-3-(acetoxymethyl)butyl]-2-aminopurine (8.19 g) in a 90% yield.1H-NMR (CDC13) (d, ppm): 1.87-1.95 (m, 3H, CH and CH2) 2.00 (s, 6H, 2xCH3) 4.07 (d, 4H, 2xCH2O) 4.18 (t, 2H, CH2N) 5.17 (br, 2H, NH2) 7.72 (s, 1H, CH) 8.63 (s, 1H, CH).13C-NMR (CDC13) (d, ppm): 20.82 (2xCH3) 28.83 (CH2) 34.95 (CH) 40.79 (CH2N) 63.65 (2xOCH2) 128.21 () 142.16 (C) 149.90 (CH) 153.20 (C) 159.95 (C) 170.70 (2xCO). EI-MS: 321 m/z (M+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping