| 54% |
at 100℃; for 48 h; Molecular sieve |
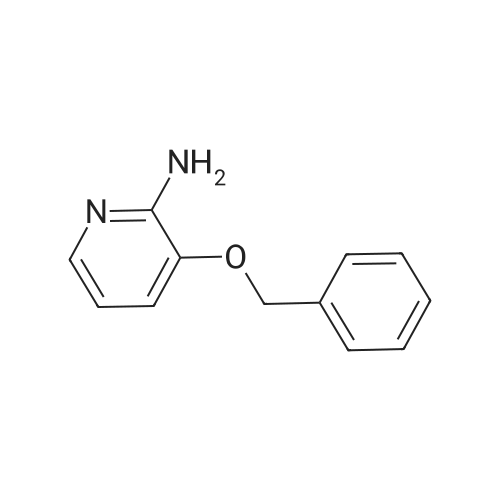
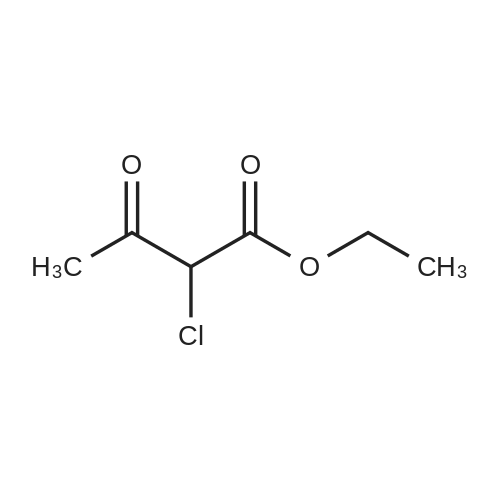
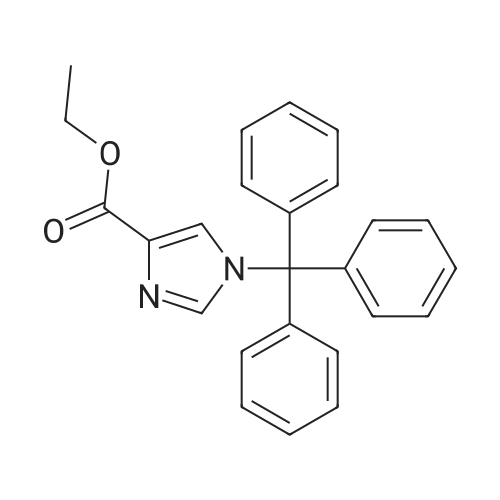
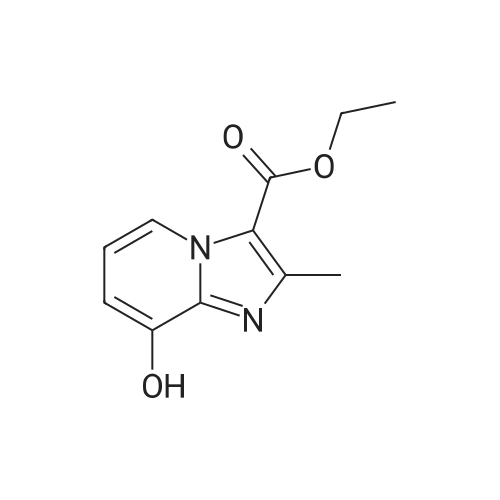
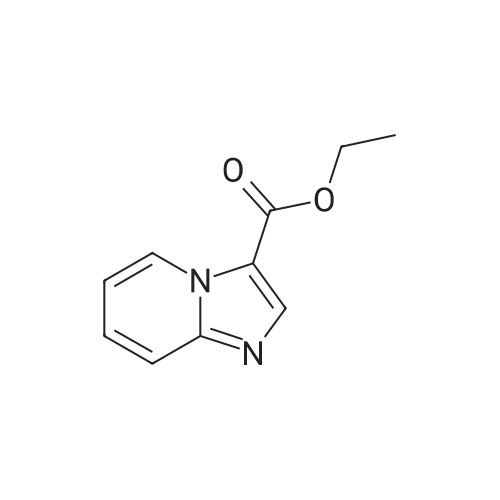
Example 1A
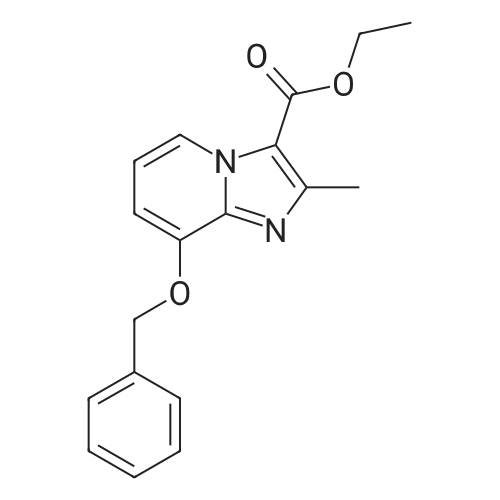
Ethyl 8-(benzyloxy)-2-methylimidazo[1,2-a]pyridine-3-carboxylate
25 g (124.8 mmol) of 2-amino-3-benzyloxypyridine were dissolved in 781 ml of ethanol, 102.7 g (624.2 mmol) of ethyl 2-chloroacetoacetate and two tablespoons of 4 A molecular sieve were added, and the reaction mixture was then heated at reflux (bath temperature 100° C.) for 2 days.
The mixture was concentrated, and excess ethyl 2-chloroacetoacetate was removed on a rotary evaporator with dry ice cooling.
The residue was purified by silica gel chromatography (mobile phase cyclohexane:ethyl acetate gradient 9:1, 4:1).
This gave 20.81 g of the target compound (54percent of theory, purity 99percent).
LC-MS (Method 2): Rt=1.12 min
MS (ESpos): m/z=311 (M+H)+
1H NMR (400 MHz, DMSO-d6): δ=1.35 (t, 3H), 2.59 (s, 3H), 4.34 (q, 2H), 5.32 (s, 2H), 7.01-7.09 (m, 2H), 7.33-7.48 (m, 3H), 7.52 (d, 2H), 8.81-8.86 (m, 1H). |
| 54% |
at 100℃; for 48 h; Molecular sieve |
Example 29A
Ethyl 8-(benzyloxy)-2-methylimidazo[1,2-a]pyridine-3-carboxylate
25 g of 2-amino-3-benzyloxypyridine (124.8 mmol, 1 equivalent) were dissolved in 781 ml of ethanol, and 102.7 g of ethyl 2-chloroacetoacetate (624.2 mmol, 5 equivalents) and 15 g of 4 ? molecular sieve were added.
The mixture was heated at reflux for 2 d (bath temperature 100° C.).
The mixture was then concentrated and excess ethyl 2-chloroacetoacetate was distilled off on a rotary evaporator with dry ice-cooling.
The residue was purified by silica gel chromatography (mobile phase cyclohexane:ethyl acetate 9:1, 4:1).
This gave 20.81 g of the title compound (54percent of theory).
LC-MS (Method 1): Rt=1.12 min
MS (ESpos): m/z=311 (M+H)+
1H NMR (400 MHz, DMSO-d6): δ=1.35 (t, 3H), 2.59 (s, 3H), 4.34 (q, 2H), 5.32 (s, 2H), 7.01-7.09 (m, 2H), 7.33-7.48 (m, 3H), 7.52 (d, 2H), 8.81-8.86 (m, 1H). |
| 54% |
at 100℃; for 48 h; Molecular sieve |
Example 23A
Ethyl 8-(benzyloxy)-2-methylimidazo[1,2-a]pyridine-3-carboxylate
25 g (124.8 mmol) of 2-amino-3-benzyloxypyridine were dissolved in 781 ml of ethanol, 102.7 g (624.2 mmol) of ethyl 2-chloroacetoacetate and two table spoons of 4 A molecular sieve were added and the reaction mixture was then heated at reflux (bath temperature 100° C.) for 2 days.
The mixture was concentrated and excess ethyl 2-chloroacetoacetate was distilled off on a rotary evaporator using dry ice cooling.
The residue was purified by silica gel chromatography (mobile phase: cyclohexane/ethyl acetate gradient-9/1, 4/1).
This gave 20.81 g of the target compound (54percent of theory, purity 99percent).
LC-MS (Method 2): Rt=1.12 min
MS (ESpos): m/z=311 (M+H)+
1H-NMR (400 MHz, DMSO-d6): δ=1.35 (t, 3H), 2.59 (s, 3H), 4.34 (q, 2H), 5.32 (s, 2H), 7.01-7.09 (m, 2H), 7.33-7.48 (m, 3H), 7.52 (d, 2H), 8.81-8.86 (m, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping