| 100% |
With lithium diisopropyl amide; In tetrahydrofuran; hexane; water; at 20℃; for 12.25h; |
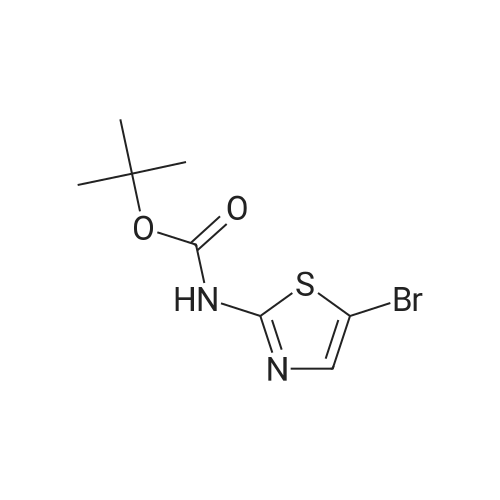
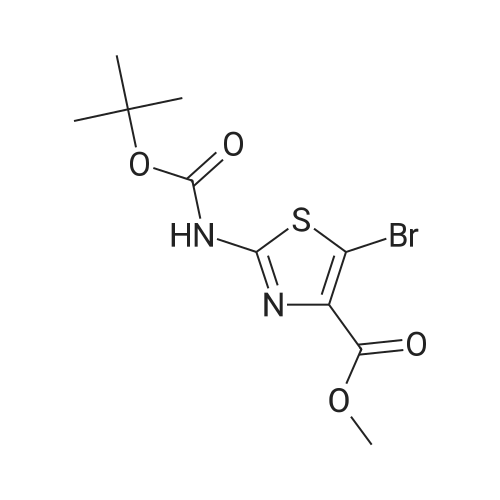
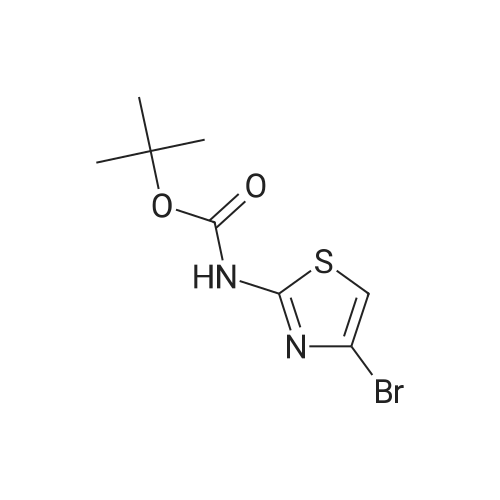
Diisopropylamine (2.3 mL, 16 mmol) was taken up in 30 mL of THF and chilled to 0 C. Butyllithium, 2.5M in hexane (6.4 mL, 16 mmol), was added to the reaction mixture, and the mixture was stirred for 20 minutes. tert-Butyl 5-bromothiazol-2-ylcarbamate (1.5 g, 5.4 mmol) was then added slowly in 8 mL of THF. After 15 minutes, approximately 2 mL of water was added, and the mixture was warmed to room temperature and stirred for 12 hours. The mixture was diluted with 30 mL of 1/2 saturated aqueous NH4Cl and transferred to a separatory funnel. The mixture was extracted twice with 5 mL of EtOAc, and the combined organic extracts were washed with brine and dried over MgSO4. Filtration and concentration under reduced pressure afforded tert-butyl 4-bromothiazol-2-ylcarbamate (1.5 g, 100% yield) as a brown solid. |
| 100% |
With n-butyllithium; diisopropylamine; In tetrahydrofuran; hexane; at 20℃; |
t-Butyl 4-bromothiazol-2-ylcarbamate: Diisopropylamine (2.3 mL, 16 mmol, Aldrich) was taken up in 30 mL of THF, and the mixture was chilled to 0 0C. Butyllithium (2.5 M in hexane (6.4 mL, 16 mmol, Aldrich)) was added to the reaction mixture, and the mixture was stirred for 20 minutes. tert-Butyl 5-bromothiazol-2- ylcarbamate (1.5 g, 5.4 mmol, prepared as shown in Scheme 2) was then added slowly in 8 mL of THF. After 15 minutes, approximately 2 mL of water was added, and the mixture was warmed to room temperature and stirred for 12 hours. The mixture was diluted with 30 mL of 1/2 saturated aqueous NH4Cl and transferred to a separatory funnel. The mixture was extracted twice with 5 mL of EtOAc, and the combined organic extracts were washed with brine and dried over MgSO4. Filtration and concentration under reduced pressure afforded tert-butyl 4-bromothiazol-2-ylcarbamate (1.5 g, 100 %) as a brown solid |
| 96% |
With lithium diisopropyl amide; In tetrahydrofuran; hexanes; water; at 0 - 7℃; for 0.6h; |
4.3 Synthesis of tert-butyl (4-bromo-1,3-thiazol-2-yl)carbamate (507) Tetrahydrofuran (160 mL) and N,N-diisopropylamine (14 mL, 97 mmol) were combined in a 3-neck 500 mL RBF equipped with a stirbar, septum, and an internal temperature probe. The resulting solution was cooled to 0.8 C., and n-butyllithium in hexanes (2.50 M, 38 mL, 95 mmol) was added slowly over ca. 5 min to produce a light yellow solution (Tint max=10 C.), which was stirred and allowed to re-cool to near 0 C. A solution of tert-butyl (5-bromo-1,3-thiazol-2-yl)carbamate, 506 (8.74 g, 31.3 mmol) in tetrahydrofuran (30.0 mL) was added dropwise to the above solution over 16 min (Tit varied from 0.9 C. to a maximum of 7 C.). The now deep brown reaction mixture was stirred for 15 min before being quenched with water (13 mL), and stirred for an additional 5 minutes. Aqueous saturated NH4Cl (250 mL) and ethyl acetate (250 mL) were added, and the layers were separated. The aqueous was extracted with ethyl acetate, and the combined organics were washed with brine, dried with MgSO4, filtered, and concentrated in vacuo. The crude material was adsorbed onto silica gel with ethyl acetate, and elute through a plug of silica gel with 2 liters of 9:1 hex:EtOAc The eluent was collected, and the solvents were removed to give 507 (8.41 g, 96%) as a colorless solid. Data for 507: 1H NMR (400 MHz, DMSO-d6) d 12.75 (br s, 1 H), 7.24 (s, 1 H), and 1.48 (s, 9 H) ppm. 13C NMR (400 MHz, CDCl3) d 160.6, 152.7 (br), 119.8, 110.6, 81.7, and 27.9 ppm. MS (ESI+) m/z (rel. intensity): 225.1 (100, M81Br-(CH3)2C=CH2+), 223.1 (100, M79Br-(CH3)2C=CH2+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping