|
With ethanol; diethylamino-sulfur trifluoride; In dichloromethane; for 12h; |
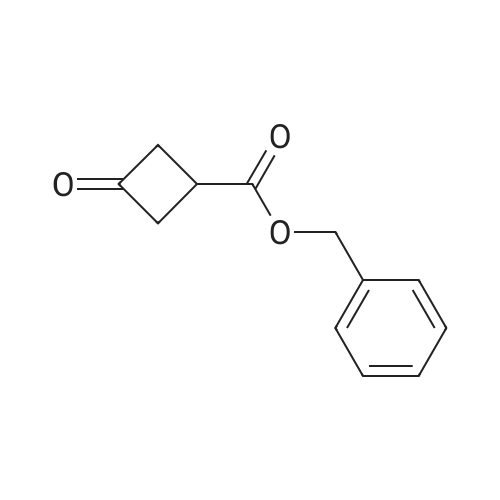
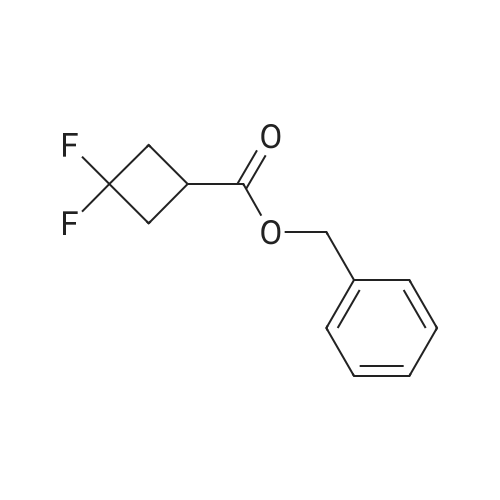
Benzyl 3-oxocyclobutanecarboxylate (2-B) (1.23 g, 6.03 mmol) was dissolved in methylene chloride (35 ml). DAST (8.0 ml, 6.03 mmol) was added under nitrogen, followed by anhydrous ethanol (0.4 ml, 7.23 mmol). The mixture was stirred for 12 hours before it was diluted with methylene chloride, washed successively with saturated sodium bicarbonate, IN aq. hydrochloric acid, and brine. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by silica gel chromatography with 93% hexane/ethyl acetate as eluent to give <n="25"/>2-C as an oil. lH NMR (500 MHz, CDCI3): delta 2.81-2.93 (4H, m), 3.01- 3.04 (IH, m), 5.20 (2H, s), 7.36- 7.42 (5H, m) ppm. |
|
With diethylamino-sulfur trifluoride; In ethanol; dichloromethane; for 12h; |
Step B:Benzyl 3-oxocyclobutanecarboxylate (2-B) (1.23 g, 6.03 mmol) was dissolved in methylene chloride (35 ml). DAST (8.0 ml, 6.03 mmol) was added under nitrogen, followed by anhydrous ethanol (0.4 ml, 7.23 mmol). The mixture was stirred for 12 hours before it was diluted with methylene chloride, washed successively with saturated sodium bicarbonate, 1N aq. hydrochloric acid, and brine. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by silica gel chromatography with 93% hexane/ethyl acetate as eluent to give 2-C as an oil. |
|
With diethylamino-sulfur trifluoride; |
Step B: Benzyl 3,3-difluorocyclobutanecarboxylate To a solution of <strong>[198995-91-4]benzyl 3-oxocyclobutanecarboxylate</strong> (1.23 g, 6.03 mmol) in DCM (35 mL) was added DAST (0.8 mL, 6.03 mmol) dropwise under nitrogen. The mixture was stirred at room temperature for 16 h and then diluted with DCM. After successive washes with saturated sodium bicarbonate, 1N aq. hydrochloride acid, and brine, the organic layer was dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by silica gel chromatography with 93% hexane/EtOAc as eluent to give the desired compound as an oil. 1H NMR (400 MHz, CDCl3): delta 7.47-7.27 (m, 5H), 5.16 (s, 2H), 3.09-2.95 (m, 1H), 2.90-2.60 (m, 4H). |
|
With diethylamino-sulfur trifluoride; In dichloromethane; at 20℃; for 16h;Inert atmosphere; |
Step B: Benzyl 3,3-difluorocyclobutanecarboxylate. To a solution of benzyl 3- oxocyclobutanecarboxylate (1.23g, 6.03 mmol) in DCM (35 mL) was added DAST (0.8 mL, 6.03 mmol) dropwise under nitrogen. The mixture was stirred at room temperature for 16 h and then diluted with DCM. After successive washes with saturated sodium bicarbonate, IN aq. hydrochloride acid, and brine, the organic layer was dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by silica gel chromatography with 93% hexane / EtOAc as eluent to give the desired compound as an oil. H NMR (400 MHz, CDC13): delta 7.47 - 7.27 (m, 5H), 5.16 (s, 2H), 3.09 - 2.95 (m, 1H), 2.90 - 2.60 (m, 4H). |
|
With diethylamino-sulfur trifluoride; In dichloromethane; at 20℃; for 16h;Inert atmosphere; |
To a solution of <strong>[198995-91-4]benzyl 3-oxocyclobutanecarboxylate</strong> (1 .23g, 6.03 mmol) in DCM (35 mL) was added DAST (0.8 mL, 6.03 mmol) dropwise under nitrogen. The mixture was stirred at room temperature for 16 hand then diluted with DCM. After successive washes with saturated sodium bicarbonate, iN aq. hydrochloride acid, and brine, the organic layer was dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by silica gel chromatography with 93% hexane EtOAc as eluent to give the desired compound as an oil. ?H NMR (400 MHz, CDC13): 7.47 -7.27 (m, 511), 5.16 (s, 211), 3.09 -2.95 (m, 111), 2.90 -2.60 (m, 411). |
|
With diethylamino-sulfur trifluoride; In dichloromethane; at 20℃; for 16h;Inert atmosphere; |
Step B: Benzyl 3, 3-difluorocyclobutanecarboxylate. To a solution of benzyl 3- oxocyclobutanecarboxylate (1 .23g, 6.03 mmol) in DCM (35 mL) was added DAST (0.8 mL, 6.03 mmol) dropwise under nitrogen. The mixture was stirred at room temperature for 16 h and then diluted with DCM . After successive washes with saturated sodium bicarbonate, IN aq. hydrochloride acid, and brine, the organic layer was dried over anhydrous sodium sulfate, filtered and concentrated. The crude product waspurifiedby silica gel chromatography with 93% hexane / EtOAc as eluent to give the desired compound as an oiL H NMR (400MHz, CDC13): delta 7.47 - 7.27 (m, 5H), 5.16 (s, 2H), 3.09 - 2.95 (m, 1H), 2.90 - 2.60 (m, 4H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping