| 59% |
With acetic acid; sodium nitrite; at 20℃; |
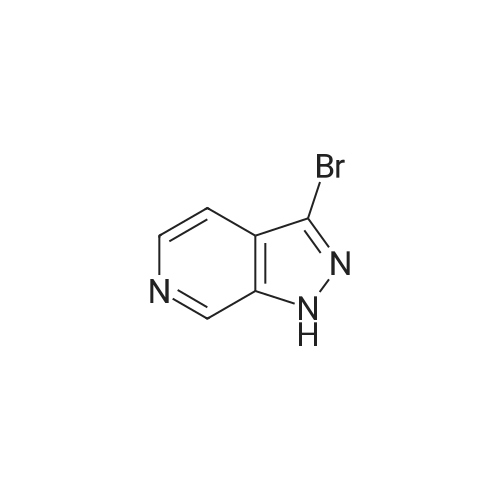
To a solutionof 27(4.00 g, 21.4 mmol) in AcOH (300 ml) was added NaNO2 (1.48 g, 21.4mmol) and stirred overnight at roomtemperature. The reaction mixture was concentrated in vacuo. The residue was diluted with EtOAc and washed with saturatedNaHCO3 aqueous solution and brine. Theorganic layer was dried over anhydrous MgSO4 and reduced underpressure. The residue was purified bysilica gel column chromatography (0-50% EtOAc in hexanes) to afford 28 as a yellow solid (2.48 g, 59%yield).1H NMR (300 MHz, CDCl3): delta ppm7.86 - 7.90 (m, 1 H), 8.09 - 8.14 (m, 1 H), 8.83 - 8.88 (m, 1 H).MS ESI/APCI Dual m/z: 198 [M+H] . |
|
|
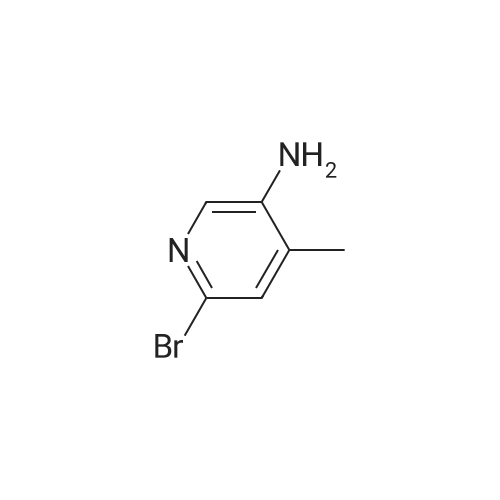
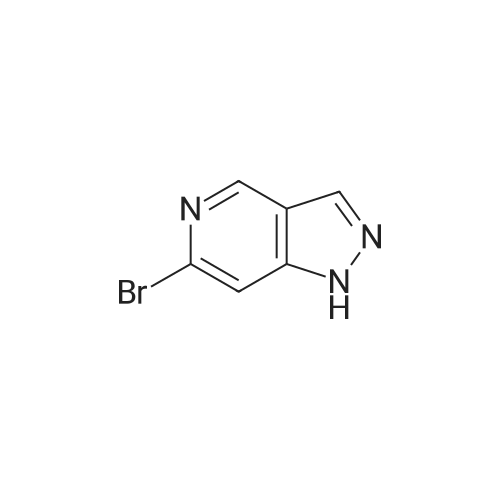
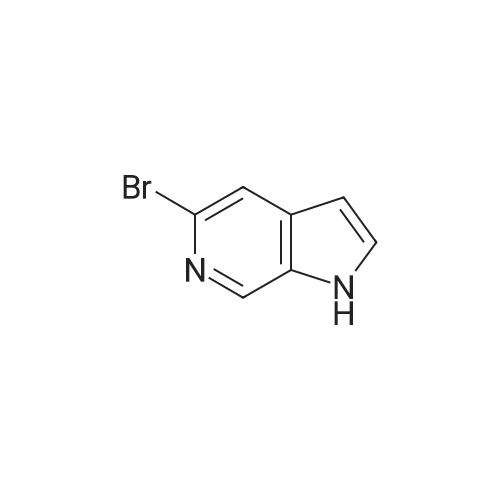
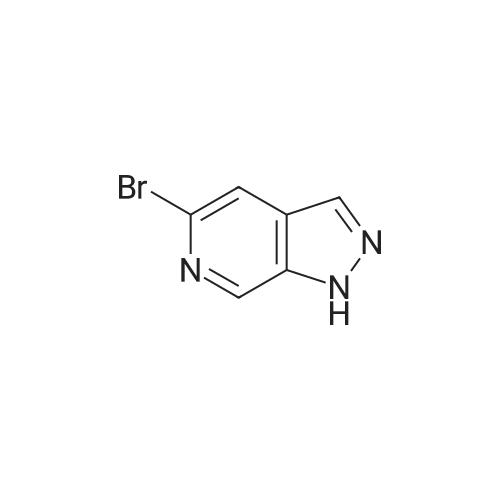
Step 2 [0611] To a suspension 6-bromo-4-methylpyridin-3 -amine (XV) (186 g, 994 mmol, 1.00 eq) and KOAc ( 1 15 g, 1.17 mol, 1.18 eq) in CHC13 (3.50 L) was added Ac20 (405 g, 3.97 mol, 3.99 eq) and the suspension was stirred at 25C for 1 h and then heated at 60-70C to reflux for an additional 2 h. After cooling the suspension to 25C, isopentyl nitrate (233 g, 1.99 mol, 2.00 eq) and 18-crown-6 (21 g, 79.5 mmol, 0.08 eq) was added and the suspension heated to reflux for 12 h. After cooling to 25C, the suspension was filtered and the filtrate was concentrated under reduced pressure to yield a residue that was treated with a suspension of potassium carbonate (450 g) in a solution of methanol and water (450 mL) at 0C for 3 h. The suspension was concentrated under reduced pressure to yield a residue that was extracted with EtOAc (1000 mL x 3) and washed with brine. The organic layer was dried over sodium sulfate, filtered and concentrated under reduced pressure to give 5-bromo-lH-pyrazolo[3,4-c]pyridine (XVI) (200 g, crude) as yellow solid. The crude product was used for the next step without any purification. NMR (DMSO- tf, 400 MHz) delta ppm 7.96 (s, 1H), 8.15 (s, 1H), 8.86 (s, 1H); ESIMS found for C6H4BrN3 mlz 198.3 (M+H). |
|
|
Step 2 [0612] To a suspension 6-bromo-4-methylpyridin-3 -amine (XV) (186 g, 994 mmol, 1.00 eq) and KOAc (115 g, 1.17 mol, 1.18 eq) in CHC13 (3.50 L) was added Ac20 (405 g, 3.97 mol, 3.99 eq) and the suspension was stirred at 25C for 1 h and then heated at 60-70C to reflux for an additional 2 h. After cooling the suspension to 25C, isopentyl nitrate (233 g, 1.99 mol, 2.00 eq) and 18-crown-6 (21 g, 79.5 mmol, 0.08 eq) was added and the suspension heated to reflux for 12 h. After cooling to 25C, the suspension was filtered and the filtrate was concentrated under reduced pressure to yield a residue that was treated with a suspension of potassium carbonate (450 g) in a solution of methanol and water (450 mL) at 0C for 3 h. The suspension was concentrated under reduced pressure to yield a residue that was extracted with EtOAc (1000 mL x 3) and washed with brine. The organic layer was dried over sodium sulfate, filtered and concentrated under reduced pressure to give 5-bromo-lH-pyrazolo[3,4-c]pyridine (XVI) (200 g, crude) as yellow solid. The crude product was used for the next step without any purification. NMR (DMSO- tf, 400 MHz) delta ppm 7.96 (s, 1H), 8.15 (s, 1H), 8.86 (s, 1H); ESIMS found for C6H4BrN3 mlz 198.3 (M+H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping