| 22% |
|
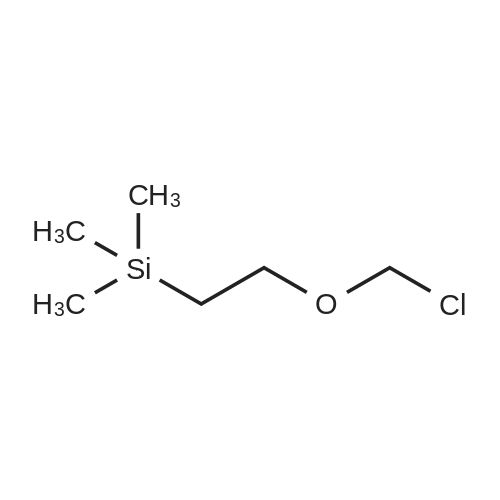
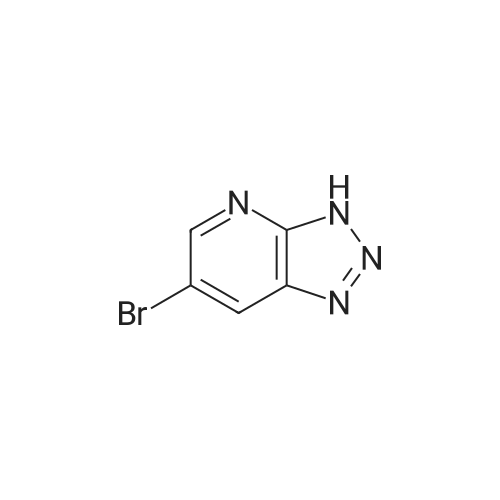
General procedure: To a stirred solution of 6-bromo-1H-i,2,3-triazolo[4,5-b]pyridine (250 mg, 1.26 mmol) in DMF (5 mL) at rt was added 60% sodium hydride in mineral oil (55 mg, 1.38 mmol) and the mixture was stirred at rt for 30 mins. (2-(Chloromethoxy)ethyl)trimethylsilane (419 mg, 2.51 mmol) was added and the mixture was stirred for 15 h. The reaction mixture was partitioned between water and EtOAc and the aqueous layer was separated and further extracted with EtOAc. The combined organic layers were dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel flash chromatography eluting with 0 - 20% EtOAc in hexanes to afford a 1:1 mixture of 6-bromo- 1 -((2- (trimethylsilyl)ethoxy)methyl)- 1H- [1,2,3 ]triazolo [4,5-b]pyridine and 6-bromo-3 -((2- (trimethylsilyl)ethoxy)methyl)-3H- [1,2,3 ]triazolo[4,5 -b]pyridine (240 mg) as an oil, which was not purified further. LC-MS (ESI) mlz 329 and 331 (M+H).v [000223] Step 2: A 1:1 mixture of N-(5 -(1,1,1 -trifluoro-2-methylpropan-2-yl)isoxazol-3 - yl)-2-(4-(l -((2-(trimethylsilyl)ethoxy)methyl)- 1H- [1,2,3 ]triazolo [4,5 -b]pyridin-6- yl)phenyl)acetamide and N-(5 -(1,1,1 -trifluoro-2-methylpropan-2-yl)isoxazol-3 -yl)-2-(4-(3 -((2- (trimethylsilyl)ethoxy)methyl)-3H- [1,2,3 ]triazolo [4,5 -b]pyridin-6-yl)phenyl)acetamide (71 mg, 40%) was obtained as a solid using a procedure analogous to that described in Step 3 of Example4, substituting the product obtained from Step 1 of this example for the 2-chloro-6,7- dimethoxyquinoxaline used in Example 4 and substituting 2-(4-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)phenyl)-N-(5 -(1,1,1 -trifluoro-2-methylpropan-2-yl)isoxazol-3 -yl)acetamide (Ref: S. Abraham et al, WO 2011022473 Al) for the 2-(2-fluoro-4-(4,4,5,5-tetramethyl-l,3,2- dioxaborolan-2-yl)phenyl)-N-(5 -(1 -(trifluoromethyl)cyclopropyl)isoxazol-3 -yl)acetamide used in Example 4. LC-MS (ESI) mlz 561 (M+H).[000224] Step 3: To a 1:1 mixture of N-(5 -(1,1,1 -trifluoro-2-methylpropan-2-yl)isoxazol-3 - yl)-2-(4-(l -((2-(trimethylsilyl)ethoxy)methyl)- 1H- [1,2,3 ]triazolo [4,5 -b]pyridin-6- yl)phenyl)acetamide and N-(5 -(1,1,1 -trifluoro-2-methylpropan-2-yl)isoxazol-3 -yl)-2-(4-(3 -((2- (trimethylsilyl)ethoxy)methyl)-3H- [1,2,3 ]triazolo [4,5 -b]pyridin-6-yl)phenyl)acetamide (71 mg, 0.127 mmol) was added trifluoroacetic acid (5 mL), and the mixture was stirred at rt for 1 h. The mixture was concentrated under reduced pressure and the residue was purified by reverse-phase preparative HPLC using a mixture of water (containing 5% CH3CN and 0.05% HCOOH) and CH3CN (containing 0.05% HCOOH) as the mobile phase and Varian Pursuit XRs diphenyl column as the stationary phase to afford 2-(4-(3H-[l,2,3]triazolo[4,5-b]pyridin-6-yl)phenyl)-N- (5 -(1,1,1 -trifluoro-2-methylpropan-2-yl)isoxazol-3 -yl)acetamide (12 mg, 22%) as a solid. ?H NMR (500 MHz, DMSO-d6) oe 11.41 (br s, 1H), 9.00 (d, J= 2.0 Hz, 1H), 8.60 (br s, 1H), 7.80 (d, J 8.5 Hz, 2H), 7.48 (d,J 8.5 Hz, 2H), 6.95 (s, 1H), 3.77 (s, 2H), 1.53 (s, 6H); LC-MS (ESI) m/z 431 (M+H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping