| 21% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium carbonate; In 1,4-dioxane; at 20 - 100℃; for 64h;Inert atmosphere; |
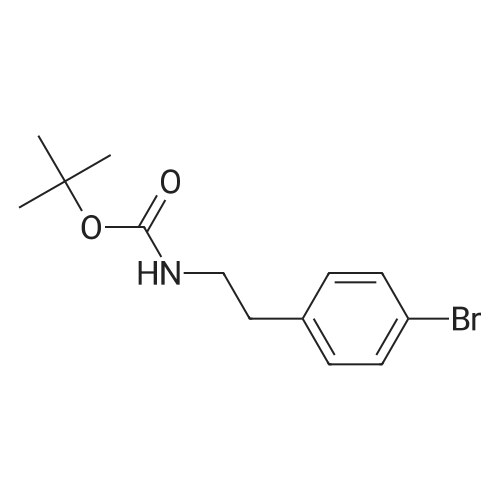
A mixture of te/f-butyl A/-[2-(4-bromophenyl)ethyl]carbamate, Intermediate 142 (4.09 g, 13.6 mmol), [4-cyanomethyl)phenyl]boronic acid (2.63 g, 16.4 mmol) and K2CO3 (5.65 g, 40.9 mmol) in 1 ,4-dioxane (105 ml) was degassed by bubbling a stream nitrogen through the mixture for 5 min. Pd(dppf)Cl2.CH2Cl2 (445 mg, 0.545 mmol) was added and degassing was continued for a further 5 min. The reaction mixture was heated at 80 C for 15 h then at 100 C for 7 h. The reaction was allowed to cool to RT then retreated with K2CO3 (3.76 g, 27.2 mmol) and degassed for 5 min. Pd(dppf)Cl2.CH2Cl2 (445 mg, 0.545 mmol) was added then the mixture was degassed for a further 5 min. The resultant mixture was heated at 100 C for 24 h then allowed to cool to RT. The reaction was retreated with K2CO3 (1.88 g, 13.6 mmol) and [4-cyanomethyl)phenyl]boronic acid (0.88 g, 5.5 mmol) then degassed for 10 min. Pd(dppf)Cl2.CH2Cl2 (445 mg, 0.545 mmol) was added then the mixture was degassed for a further 5 min. The reaction was heated at 100 C for 18 h then allowed to cool to RT. The reaction mixture was filtered then the collected solids were washed with EtOAc (50 ml). The combined filtrate was concentrated in vacuo. The residue was re- dissolved in EtOAc:heptane (1 : 1) then filtered through a silica pad. The pad was rinsed with EtOAc:heptane (1 :1 , 200 ml). The filtrate was concentrated in vacuo to afford an off- white solid (3.94 g). The silica pad was rinsed through further with EtOAc (200 ml) to afford a brown solid (1.68 g). The brown solid from the EtOAc filtrate was pre-adsorbed onto silica, then purified by flash column chromatography on a silica column (50 g). The column was eluted with EtOAc: heptane, using the following gradient (%EtOAc, column volumes): 0%, 1 CV; 0-30%, 1 1 CV; 30%, 20 CV; 30-45%, 4.5 CV; 45%, 7.5 CV; 45-50%, 1 CV; 50%, 15 CV. The desired fractions were combined and concentrated in vacuo to afford an off-white solid (1.00 g, 21 %). 1 H NMR (500 MHz, DMSO-cfe) delta 7.67 (d, J = 8.2 Hz, 2H), 7.59 (d, J = 8.2 Hz, 2H), 7.42 (d, J = 8.2 Hz, 2H), 7.28 (d, J = 8.1 Hz, 2H), 6.90 (t, J = 5.5 Hz, 1 H), 4.07 (s, 2H), 3.17 (q, J = 6.5 Hz, 2H), 2.73 (t, J = 7.4 Hz, 2H), 1.44 - 1.29 (m, 9H). LC/MS (System A): Rt = 1.27 min, UV purity = 97%. |
| 21% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium carbonate; In 1,4-dioxane; at 80 - 100℃; for 64h;Inert atmosphere; |
A mixture of teit-butyl N-[2-(4-bromophenyl)ethyl]carbamate, Intermediate 29 (4.09 g,13.6 mmol), [4-cyanomethyl)phenyl]boronic acid (2.63 g, 16.4 mmol) and K2C03 (5.65 g,40.9 mmol) in 1,4-dioxane (105 ml) was degassed by bubbling a stream nitrogen throughthe mixture for 5 mm. Pd(dppf)C12.CH2CI2 (445 mg, 0.545 mmol) was added anddegassing was continued for a further 5 mm. The reaction mixture was heated at 80 Cfor 15 h then at 100 C for 7 h. The reaction was allowed to cool to RT then retreated with K2C03 (3.76 g, 27.2 mmol) and degassed for 5 mm. Pd(dppf)C12.CH2CI2 (445 mg, 0.545 mmol) was added then the mixture was degassed for a further 5 mm. The resultant mixture was heated at 100 C for 24 h then allowed to cool to RT. The reaction wasretreated with K2C03 (1.88 g, 13.6 mmol) and [4-cyanomethyl)phenyl]boronic acid (0.88 g, 5.5 mmol) then degassed for 10 mm. Pd(dppf)C12.CH2CI2 (445 mg, 0.545 mmol) was added then the mixture was degassed for a further 5 mm. The reaction was heated at 100 C for 18 h then allowed to cool to RT. The reaction mixture was filtered then the collected solids were washed with EtOAc (50 ml). The combined filtrate wasconcentrated in vacuo. The residue was re-dissolved in EtOAc:heptane (1:1) then filtered through a silica pad. The pad was rinsed with EtOAc:heptane (1:1, 200 ml). The filtrate was concentrated in vacuo to afford an off-white solid (3.94 g). The silica pad was rinsed through further with EtOAc (200 ml) to afford a brown solid (1.68 g). The brown solid from the EtOAc filtrate was pre-adsorbed onto silica, then purified by flash columnchromatography on a silica column (50 g). The column was eluted with EtOAc:heptane, using the following gradient (%EtOAc, column volumes): 0%, 1 CV; 0-30%, 11 CV; 30%, 20 CV; 30-45%, 4.5 CV; 45%, 7.5 CV; 45-50%, 1 CV; 50%, 15 CV. The desired fractionswere combined and concentrated in vacuo to afford an off-white solid (1.00 g, 21%).1H NMR (500 MHz, DMSO-d6) O 7.67 (d, J= 8.2 Hz, 2H), 7.59 (d, J= 8.2 Hz, 2H), 7.42(d, J= 8.2 Hz, 2H), 7.28 (d, J= 8.1 Hz, 2H), 6.90 (t, J= 5.5 Hz, 1H), 4.07 (5, 2H), 3.17(q, J = 6.5 Hz, 2H), 2.73 (t, J = 7.4 Hz, 2H), 1.44 - 1.29 (m, 9H).LC/MS (System A): R = 1.27 mi UV purity = 97%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping