| 96% |
With hydrogenchloride; In ethanol; |
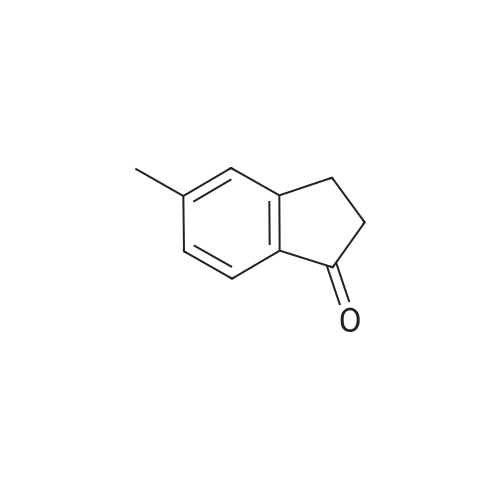
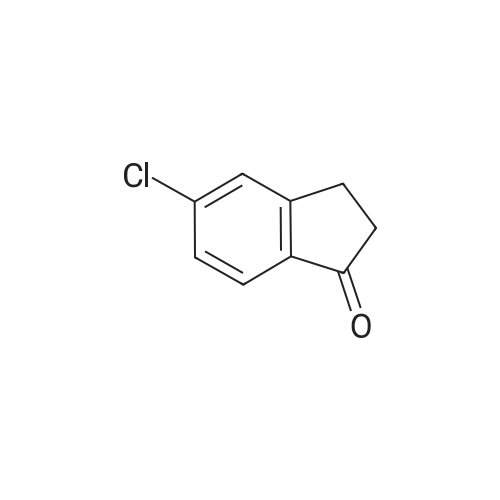
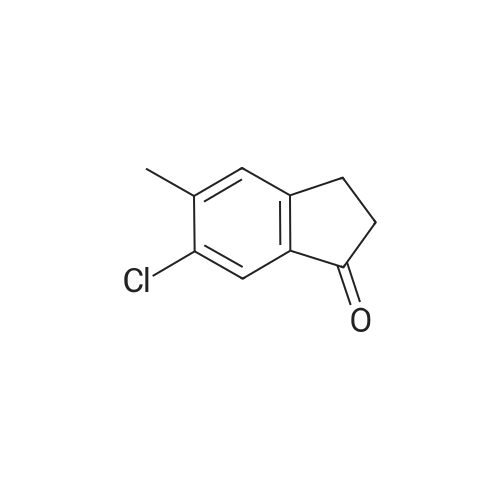
1.6a Preparation of ethyl 2-(6-chloro-5-methyl-1-oxo-2,3-dihydro-1H-inden-2-yl)-2-oxoacetate Metal sodium (0.17 g, 7.5 mmol) was added in small pieces to absolute ethanol (3.5 ml) and the mixture left under stirring until complete solubilization. Diethyloxalate (0.51 ml, 3.75 mmol) was added to the alcohol solution, followed by a dropwise addition of a solution of <strong>[919078-00-5]6-chloro-5-methylindan-1-one</strong> (12.21 mmol) in absolute ethanol (27 ml). The reaction mixture was stirred at room temperature for 9 hours. The reaction was stopped by pouring the liquid phase on a mixture of ice and HCl 1N, followed by extraction with chloroform (3*15 ml). The combined extracts were washed with water, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure. The compound ethyl 2-(6-chloro-5-methyl-1-oxo-2,3-dihydro-1H-inden-2-yl)-2-oxoacetate was isolated as an orange oil (96% yield), having an analytical grade purity. Rf=0.21 (petroleum ether/ethyl acetate 9/1 v/v); IR (nujol) (λ=cm-) 3440, 1730, 1680; 1H-NMR (CDCl3) δ 1.43 (t, 3H, J=7.2 Hz); 2.49 (s, 3H); 3.92 (s, 2H); 4.42 (q, 2H, J=7.2 Hz); 7.42 (s, 1H); 7.82 (s, 1H); 13.20 (bs, 1H). Anal. calc. for C14H13ClO4: C, 59.90; H, 4.67; Cl, 12.63. Found: C, 58.10; H, 4.71; Cl, 12.67. |
| 96% |
With sodium; In ethanol; at 20℃; |
1.6a Preparation of ethyl 2-(6-chloro-5-methyl-1-oxo-2,3-dihydro-1H-inden-2-yl)-2-oxoacetate Metal sodium (0.17 g, 7.5 mmol) was added in small pieces to absolute ethanol (3. 5 ml) and the mixture left under stirring until complete solubilization. Diethyloxalate (0.51 ml, 3.75 mmol) was added to the alcohol solution, followed by a drop-wise addition of a solution of <strong>[919078-00-5]6-chloro-5-methylindan-1-one</strong> (12.21 mmol) in absolute ethanol (27 ml). The reaction mixture was stirred at room temperature for 9 hours. The reaction was stopped by pouring the liquid phase on a mixture of ice and HCl 1N, followed by extraction with chloroform (3*15 ml). The combined extracts were washed with water, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure. The compound ethyl 2-(6-chloro-5-methyl-1-oxo-2,3-dihydro-1H-inden-2-yl)-2-oxoacetate was isolated as an orange oil (96% yield), having an analytical grade purity. Rf=0.21 (petroleum ether/ethyl acetate 9/1 v/v); IR (nujol) (λ=cm-1) 3440, 1730, 1680; 1H-NMR (CDCl3) δ 1.43 (t, 3H, J=7.2 Hz); 2.49 (s, 3H); 3.92 (s, 2H); 4.42 (q, 2H, J=7.2 Hz); 7.42 (s, 1H); 7.82 (s, 1H); 13.20 (bs, 1H). Anal. calc. for C14H13ClO4: C, 59.90; H, 4.67; Cl, 12.63. Found: C, 58.10; H, 4.71; Cl, 12.67. |
| 96% |
With ethanol; sodium; at 20℃; for 9.0h; |
Metal sodium (0.17 g, 7.5 mmol) was added in small pieces to absolute ethanol (3.5 ml) and the mixture left under stirring until complete solubilization. Diethyloxalate (0.51 ml, 3.75 mmol) was added to the alcohol solution, followed by a dropwise addition of a solution of <strong>[919078-00-5]6-chloro-5-methylindan-1-one</strong> (12.21 mmol) in absolute ethanol (27 ml). The reaction mixture was stirred at room temperature for 9 hours. The reaction was stopped by pouring the liquid phase on a mixture of ice and HCl 1N, followed by extraction with chloroform (3 x 15 ml). The combined extracts were washed with water, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure. The compound ethyl 2-(6-chloro-5-methyl-1-oxo-2,3-dihydro-1H-inden-2-yl)-2-oxoacetate was isolated as an orange oil (96% yield), having an analytical grade purity. Rf=0.21 (petroleum ether/ethyl acetate 9/1 v/v); IR (nujol) (λ = cm-1) 3440, 1730, 1680; 1H-NMR (CDCl3) δ 1.43 (t, 3H, J = 7.2 Hz); 2.49 (s, 3H); 3.92 (s, 2H); 4.42 (q, 2H, J = 7.2 Hz); 7.42 (s, 1H); 7.82 (s, 1H); 13.20 (bs, 1H). Anal. calc. for C14H13ClO4: C, 59.90; H, 4.67; Cl, 12.63. Found: C, 58.10; H, 4.71; Cl, 12.67 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping