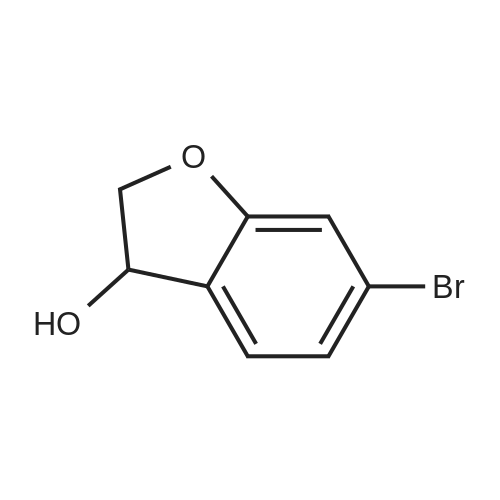
| 88.5% |
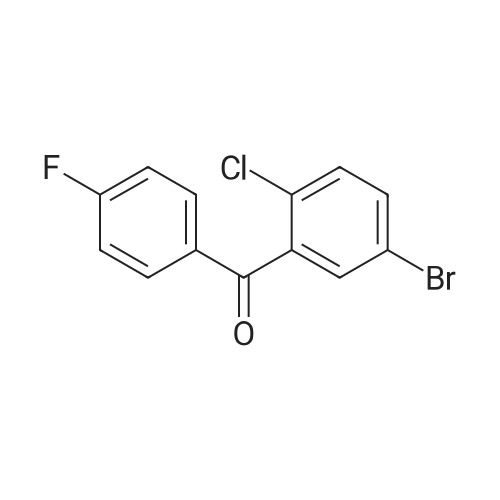
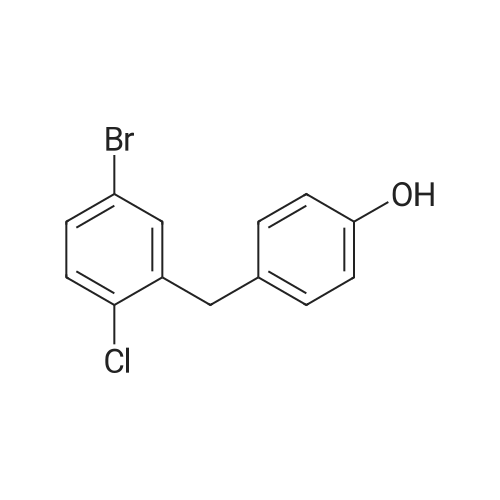
With aluminum (III) chloride; sodium tetrahydroborate; In tetrahydrofuran; at 0 - 60℃; for 24.5h; |
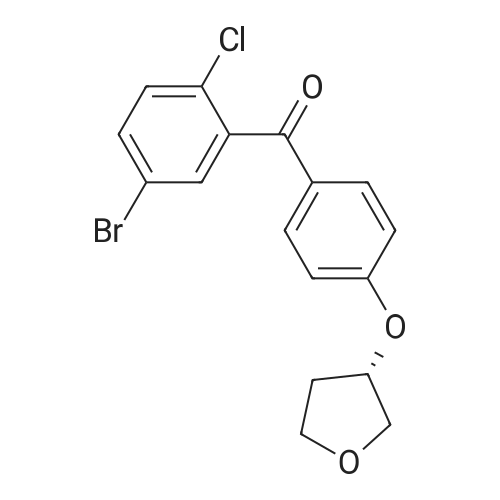
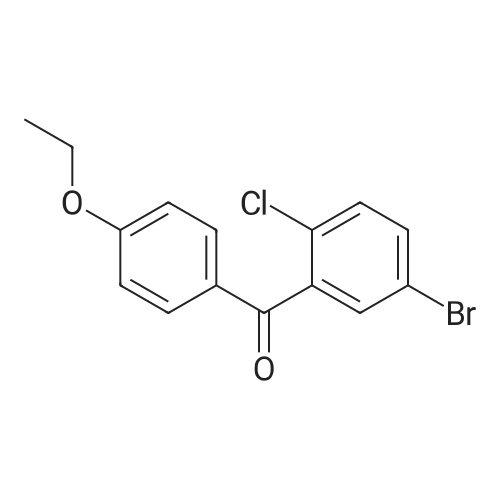
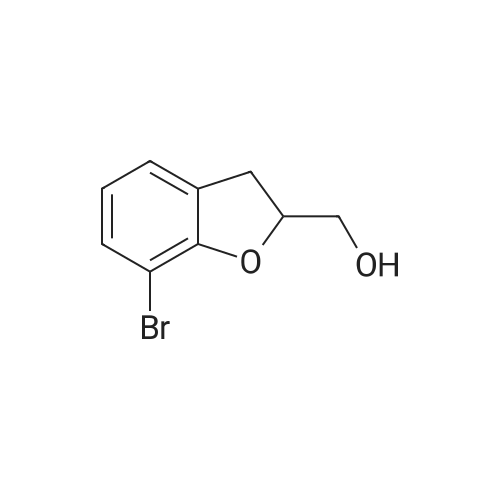
The solid obtained above (27.7 g, 72.6 mmol) was added to tetrahydrofuran (150 mL) and dissolved to dissolve.The liquid was cooled to 0 to 10 C. Then, sodium borohydride (4.14 g, 108.9 mmol, 1.5 eq) was added to the cooled reaction mixture, which was then batched.Aluminum trichloride (14.5 g, 108.9 mmol, 1.5 eq) was added. The resulting reaction mixture is warmed to room temperature and stirred for half an hour,Thereafter, the temperature was raised to 50 to 60 C and stirred for 24 hours. After the TLC detects that the starting point has substantially disappeared, the reaction mixture is cooled to 0 to 10 C.Then, water (50 mL) was slowly added dropwise to the reaction mixture to quench the reaction. Add ethyl acetate (200 mL×2) to the reaction quenching solution.Two times, the extracts were combined and concentrated to dryness under reduced pressure at 40-50 C. The obtained solid was used in methanol/water (1:1 by volume,100mL) was suspended and stirred at 40-50 C for 2 hours, then cooled to room temperature and stirred for 1 hour, and finally dropped to 0-10 C and stirred for 2 hours.Time. Filtration gave 23.6 g of a white solid, yield 88.5%. |
| 84% |
With aluminium trichloride; potassium borohydride; In tetrahydrofuran; at 0 - 20℃; for 0.5h; |
A three-necked flask was charged with 4a (38.16 g, 100 mmol)And tetrahydrofuran (304 mL),After stirring, potassium borohydride (10.79 g, 200 mmol) was added,Cooling to 0 ~ 5 ,Aluminum trichloride (13.34 g, 100 mmol) was added in portions,Add slowly after the rise to room temperature for 30 minutes,Reheat the reaction overnight.The reaction was cooled to room temperature and quenched by adding 0.5 mol / L dilute hydrochloric acid (190 mL)The aqueous phase was extracted twice with ethyl acetate (190 mL)The organic phase saturated brine was washed twice (190 mL)Dried over sodium sulfate, concentrated and recrystallized from ethanol to give compound 5a (30.88 g, 84%).The reaction solvent tetrahydrofuran in Example 5 can be replaced by dichloromethane, 1,2_dichloroethane, toluene or 2-methyltetrahydrofuran. |
| 81.8% |
With aluminum (III) chloride; potassium borohydride; In tetrahydrofuran; at 20℃; for 6h; |
Add 247 g of compound of formula 4 to the reaction flask,2L of tetrahydrofuran. Reduce the temperature to below 20 C.159 g of potassium borohydride was added at a controlled temperature.After the addition was completed, 193 g of anhydrous aluminum trichloride was added in batches at a temperature controlled below 20 C. After the addition, the reaction was refluxed for 6 hours.The TLC test was complete. The reaction solution was poured into ice water. Dilute hydrochloric acid was added dropwise. It was extracted with ethyl acetate, dried and concentrated to obtain a residue, which was purified with ethanol to obtain 193.5 g of a white compound of Formula 5 in a yield of 81.8%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping