| 57.8% |
|
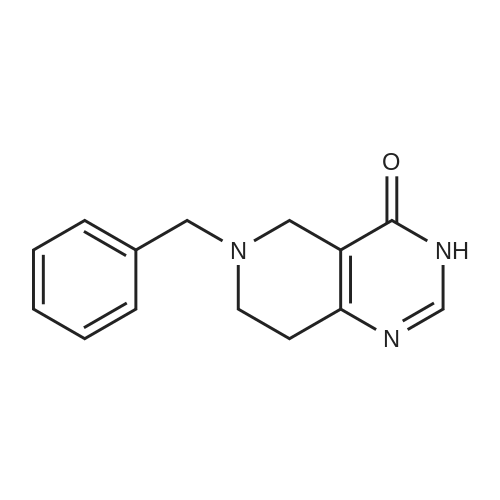
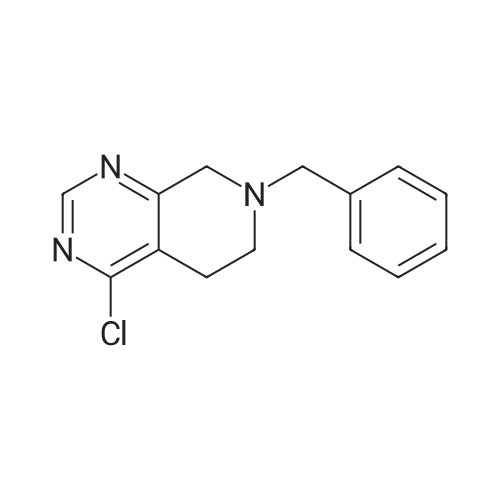
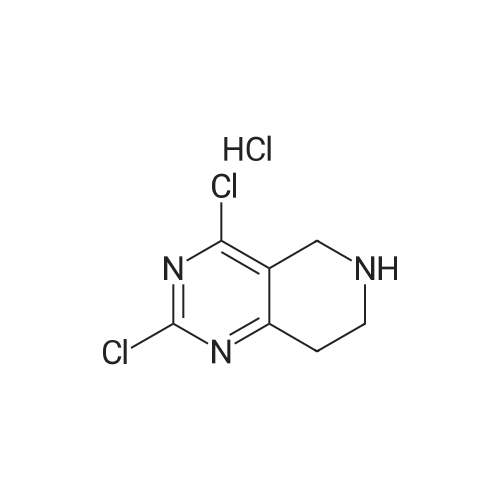
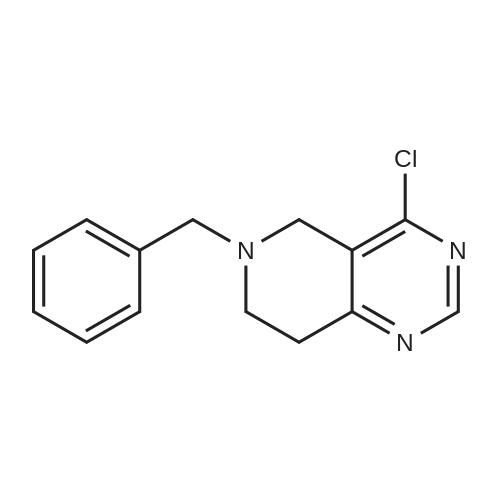
INTERMEDIATE 26-Benzyl-4-chloro-5,6,7,8-tetrahydropyrido [4,3-d] pyrimidine[00274] A mixture of <strong>[109229-22-3]6-benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4(3H)-one</strong> (5.0 g,0.02 mol), phosphoryl chloride (3.30 mL, 0.035 mol) and acetonitrile (80 mL) and DMF (catalytic amount) was heated at 70 0C for 1 hour. The mixture was concentrated in vacuo and the remaining black residue was taken up in dichloroniethane (250 mL) and poured over ice. The mixture was carefully neutralized with the addition of solid sodium bicarbonate. The organic layer was separated and dried over sodium sulfate and concentrated in vacuo. The mixture was purified by silica gel column with EtOAc/hexane (0-100%) to yield the title compound as a yellow oil (3 g, 57.8%). MS: 260 [M+l]+; 1H NMR (400 MHz, DMSO-d6): 8.80 (s, IH), 7.40-7.24 (m, 5H), 3.76 (s, 2H), 3.57 (s, 2H), 2.92 (t, 2H), 2.80 (t, 2H). |
|
With trichlorophosphate;N,N-dimethyl-formamide; In acetonitrile; for 3.0h;Reflux; |
A mixture of <strong>[109229-22-3]6-benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4(3H)-one</strong> 1 from Intermediate I, step A(5.0 g, 0.02 mol), phosphorous oxychloride (3.30 mL, 0.035 mol) and acetonitrile (80 mL) and DMF (catalytic amount) was heated at reflux for 3 hours. Then the solvents were reduced in vacuum and the remaining black residue was taken up in dichloromethane (250 mL) and poured over ice. The mixture was carefully neutralized with the addition of solid sodium bicarbonate to pH~8. The layers were separated and the organic was washed with brine, dried over sodium sulfate and concentrated. The residue was purified by column chromatography to yield compound 2 as a yellow oil (3 g). MS (ESEI): 242.1 [M+l]+ ] NMR (DMSO-d6): delta 8.80 (s, 1Eta).7.40-7.24 (m, 5H), 3.76 (s, 2H), 3.57 (s, 2H), 2.92 (t, 2H), 2.80 (t, 2H). |
|
|
A mixture of 6-benzyl-5f6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4(3H)-one 1 from Intermediate I, step A (5.0 g, 0.02 mol), phosphorous oxychloride (3.30 mL, 0.035 mol) and acetonitrile (80 mL) and DMF (catalytic amount) was heated at reflux for 3 hours. Then the solvents were reduced in vacuum and the remaining black residue was taken up in dichloromethane (250 mL) and poured over ice. The mixture was carefully neutralized with the addition of solid sodium bicarbonate to pH~8. The layers were separated and the organic was washed with brine, dried over sodium sulfate and concentrated. The residue was purified by column chromatography to yield compound 2 as a yellow oil (3 g). MS (ESEI): 242.1 [M+lf ? NMR (DMSO-d6): 8 8.80 (s, 1Eta).7.40-7.24 (m, 5H), 3.76 (s, 2H), 3.57 (s, 2H), 2.92 (t, 2H), 2.80 (t, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping