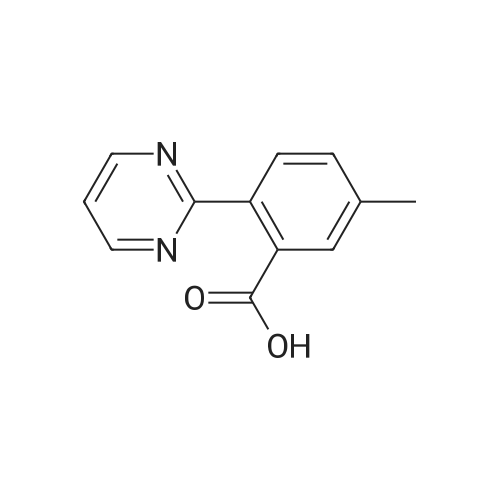
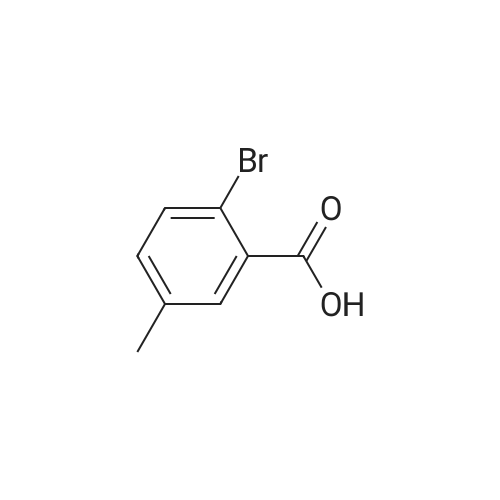
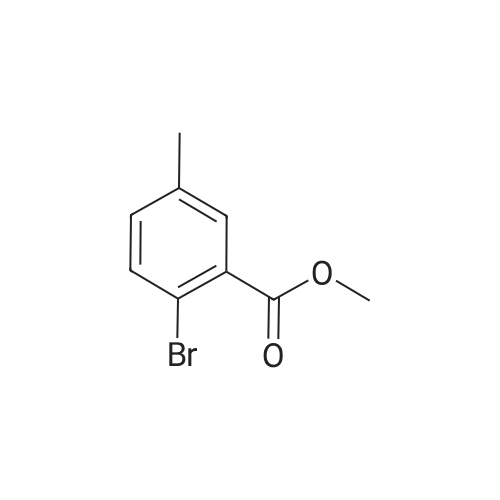
| 92% |
With acetyl chloride; at 0 - 20℃; |
Acetyl chloride (1.66 ml, 23.3 mmol) was added to a stirred solution of 2-bromo-5- methylbenzoic acid (2.00 g, 9.30 mmol) in MeOH (50 ml) at 0C. The reaction was then stirred at r.t. over night. More acetyl chloride (1 ml, 14.0 mmol) was added and the reaction was stirred for 24 hours. The solvent was removed in vacuo and the crude material was dissolved in Et02 (-100 ml) and washed with 0.5 M NaOH. The organic phase was dried over MgS04 and removed in vacuo. Yield: 1.97 g (92%). 1H NMR (400 MHz, DMSO-i?): delta 2.33 (s, 3H), 3.94 (s, 3H), 7.13 (d, 1H), 7.52 (d, 1H), 7.60 (s, 1H) |
| 92% |
|
Step 1: Preparation of methyl 2-bromo-5-methylbenzoate To a solution of <strong>[6967-82-4]2-bromo-5-methylbenzoic acid</strong> (215 mg, 1.0 mmol) in DCM (4 mL) and DMF (1 drop) at 0 C. was slowly added oxalyl chloride (0.13 mL, 1.5 mmol). The reaction mixture was stirred at rt for 1 h and to it was added additional oxalyl chloride (0.13 mL, 1.5 mmol). The reaction mixture was stirred for 1 h, added with MeOH (2 mL) and stirred for 6 h. The mixture was treated with Na2CO3 to pH of 9 and extracted with DCM (2*10 mL). The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography over silica gel eluted with PE-EA (15:1) to give methyl 2-bromo-5-methylbenzoate (210 mg; yield 92%) as colorless oil. 1H NMR (400 MHz, CDCl3): delta 7.60 (d, J=1.6 Hz, 1H), 7.53 (d, J=8.8 Hz, 1H), 7.15 (q, J=3.2 Hz, 1H), 3.92 (s, 3H), 2.33 (s, 3H) ppm. |
| 60% |
With dimethyl sulfate; sodium hydroxide; for 0.5h;Reflux; |
Dimethyl sulfate (2.2 mL, 21.1 mmol)was slowly dropped under stirring into a solution of <strong>[6967-82-4]2-bromo-5-methylbenzoic acid</strong> (3.78 g,17.6 mmol) in 22mLmethanolic NaOH. Themixturewas refluxed for 30min.After cooling,waterwas added and the solutionwas 3×extracted with diethyl ether. The combined extractswere washed twice with 5% aq NaHCO3 solution then with satd. NaCl solution and finallywith H2O. After drying over Na2SO4, the solvent was removed and the residue was purifiedby CC (Kieselgel, CH2Cl2). Yield: 2.4 g (10.5 mmol, 60%) light-yellow liquid. IR: nu =2952, 1736 (C O), 1435, 1300, 1252, 1205, 1030. 1H NMR (in agreement with lit.11): delta= 2.33 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 7.13 (dd, 1H, J = 2.0 Hz/8.0 Hz, 4-H), 7.52 (d,1H, J = 8.0 Hz, 3-H), 7.59 (d, 1 H, J = 2.0 Hz, 6-H). 13C NMR (in agreement with lit.11):delta = 20.74 (OCH3), 52.40 (ArCH3), 118.23 (Cq, ar), 131.81, 131.87, 133.42, 134.06 (CHar),137.25 (Cq,ar), 166.29 (C O). MS: m/z (%) = 230 (37) [M]+, 228 (38) [M]+, 199(94) [M- OCH3]+, 197 (100) [M - OMe]+, 171 (23) [M - CO2CH3]+, 169 (25) [M - CO2CH3]+,91 (14) [C7H7]+, 90 (48), 89 (34), 63 (15). C9H9BrO2 (229.08): calcd. C 47.19, H 3.96, Br34.88, found C 47.01, H 3.97, Br 34.85. |
|
With sulfuric acid; In water; for 16h;Heating / reflux; |
Example 16; Synthesis of <strong>[6967-82-4]2-bromo-5-methyl-benzoic acid</strong> methyl ester (Method 16.) 1 mL H2SO4 is added to <strong>[6967-82-4]2-bromo-5-methyl-benzoic acid</strong> (7.5 g, 70 mmol) in 75 mL methanol. The reaction mixture is refluxed 16 hours and cooled to room temperature. 100 mL saturated NaHCO3 (aq) is added. Methanol is removed in vacuo. The resulting mixture is extracted with ethyl acetate. The organic phase is washed with brine, dried with MgSO4 and concentrated in vacuo to give the title compound. |
|
With sulfuric acid; for 3h;Reflux; |
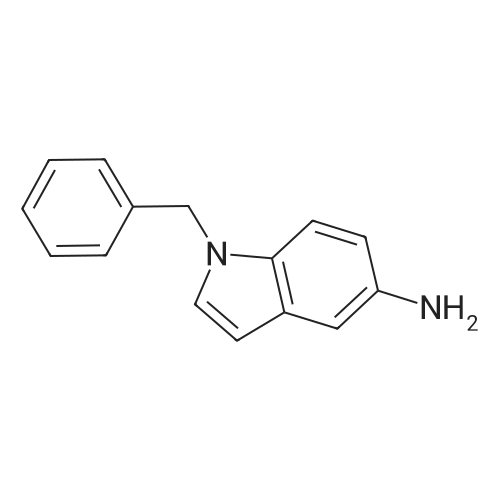
Example 205] (1250) (1251) To the solution of 108 mg of <strong>[6967-82-4]2-bromo-5-methylbenzoic acid</strong> in 10 mL of methanol, 0.5 mL of concentrated sulfuric acid was added, and the resultant was heated at reflux for three hours. The reaction mixture was cooled to room temperature, and a saturated aqueous sodium bicarbonate solution and ethyl acetate were then added thereto. The organic layer was separated and dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to give methyl 2-bromo-5-methylbenzoate. (1252) To the obtained methyl 2-bromo-5-methylbenzoate, 110 mg of 1-benzyl-1H-indol-5-amine, 10 mg of tris(dibenzylideneacetone)dipalladium(0), 18 mg of 4,5'-bis(diphenylphosphino)-9,9'-dimethylxanthene, 342 mg of cesium carbonate and 4 mL of toluene were added, and the resultant was stirred at 190C for one hour and 30 minutes using microwave equipment. After cooling the reaction mixture to room temperature, the insoluble matter was filtered off and the solvent was distilled off under reduced pressure. The obtained residue was purified by silica gel column chromatography (gradient elution with hexane:ethyl acetate = 100:0-75:25) to give methyl 2-((1-benzyl-1H-indol-5-yl)amino)-5-methylbenzoate as a yellow oil. MS (ESI, m/z): 371 (M+H)+. |
| 5.5 g |
In water; at 70℃; for 24h; |
In a 250 mL single-mouth flask, 5.00 g (23.26 mmol) of Compound 1 and 6.64 g (28.99 mmol) of Compound 2 were added, 150 mL of methanol was added, and the water separator was installed and refluxed at 70 for 24 hours to stop the reaction. After spin-drying the solvent, the reaction was washed with water several times and extracted with ethyl acetate. The ethyl acetate layer was purified by rotary column chromatography to obtain 5.5 g of compound 2 as an oil |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping