| 89% |
With triethylamine; In dichloromethane; at 50℃; for 2h; |
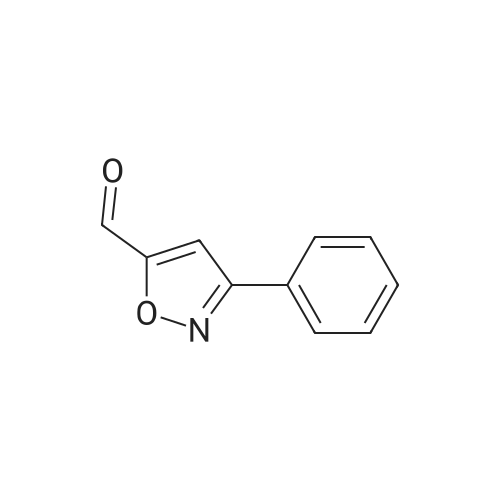
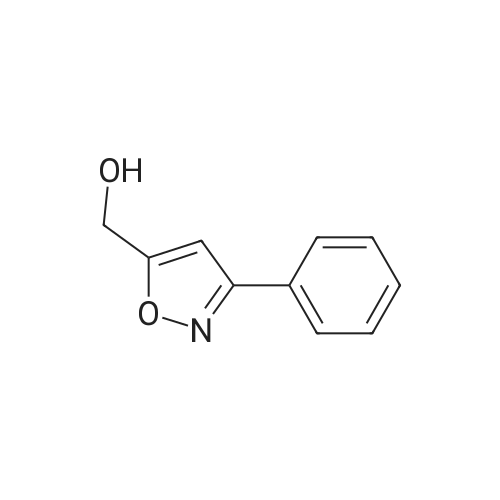
To a stirred solution of 7 (10 mg, 0.06 mmol) in dichloromethane (DCM) (0.2 ml) was addedpropargyl alcohol (5 mul, 0.09 mmol) and Et3N (13 mul, 0.09 mmol) at room temperature. Then, themixture was heated to 50 C for 2 h. After the removal of the solvent, the residue was purifiedthrough a silica chromatography column (petroleum ether : EtOAc 3 : 1) to afford the product (8)as white solid (10 mg, 89.0 %). |
|
With (1,10-phenanthroline)bis(triphenylphosphine)copper(I) nitrate; In water; at 65℃;Microwave irradiation; Green chemistry; |
General procedure: In a round bottomed flask, a mixture of benzaldehyde 1a (0.027 g,0.25 mmol, 1.0 equiv) and NaOH (0.020 g, 0.50 mmol, 2.0 equiv) was added toa solution of hydroxylamine hydrochloride (0.0177 g, 0.25 mmol, 1.0 equiv) containing 5 mL of H2O. The reaction mixture was irradiated under microwave heating at 210W for 5 min at 65 C. The progress of the reaction was monitored by TLC. After completion, N-chlorosuccinimide (0.034 g, 0.25 mmol, 1.0 equiv) was added in small proportions for over 2 min, followed by microwave irradiation at same power for 2 min. After completion, phenylacetylene 3a (0.0255 g, 0.25 mmol, 1.0 equiv) and [Cu(phen)(PPh3)2]NO3 catalysts (0.0042 g, 2 mol%) was immediately added to the reaction mixture. Subsequently, the reaction mixture was irradiated under microwave for 5 min. The reaction progress was monitored by TLC. After completion, the reaction mixture was cooled to room temperature and extracted with ethyl acetate (10 mL, twice). The combined organic layer was dried over anhydrous MgSO4. The combined filtrate was subjected to evaporation to obtain the crude compound, which was purified over silica gel column (60 - 120 mesh) using 1% ethyl acetate in hexane as eluent to obtain the corresponding 3,5-diphenylisoxazoles 4a as the product. |
|
With sodium hydrogencarbonate; copper(II) sulfate; sodium L-ascorbate; In water; N,N-dimethyl-formamide; at 30℃; for 0.166667h;Microwave irradiation; |
General procedure: The compounds were synthesised based on procedure reportedin the literature [38]. For the preparation of the imidoyl chloride, N-chlorosuccinimide (0.1 mmol) was slowly added to a solution of analdoxime (0.105 mmol) in DMF (1 mL) and the reaction was stirreduntil the starting material was not visible on the TLC analysis. After,the reaction was diluted with brine (15 mL), extracted with ethylether (3 x 10 mL), dried over Na2SO4, concentrated under vacuumand utilized without any purification in the next step. Following,propargylic alcohol (0.105 mmol), copper (II) sulphate (2 mol%),sodium ascorbate (10 mol%), sodium bicarbonate (0.4 mmol) and4mL of H2O:t-BuOH were added to the product obtained in the firstpart, and the reaction was further stirred for 4 h. Next, the reactionwas diluted with brine (15 mL), extracted with ethyl acetate(3 x 10 mL), dried over Na2SO4, concentrated under vacuum andthe crude extract was purified by flash column chromatography onsilica (hexane:ethyl acetate 6:4) yielding the expected product.After purification, they were compared via TLC analysis to therespecting compounds synthesized under microwave irradiation,when this comparison was desired, and characterized by NMR andmass spectrometry. 4.1.3. Structural characterization of compounds 6e314.1.3.1. 5-Hydroxymethyl-3-phenylisoxazole (6). Yield: 72%. YieldMW: 77%. Mp: 48.8-49.5 C. 1H NMR (300 MHz, Methanol-d4): delta 7.78-7.85 (m, 2H, Ar), 7.43-7.50 (m, 3H, Ar), 6.76 (s, 1H, Isoxazole),4.71 (s, 2H, CH2). 13C NMR (75 MHz, Methanol-d4):delta 171.7, 162.5, 130.1, 128.9, 128.8, 126.8, 100.0, 56.7. HRMS (ESITOF)m/z: [M+H]+ Calcd for C10H10NO2 176.0712; Found 176.0699 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping