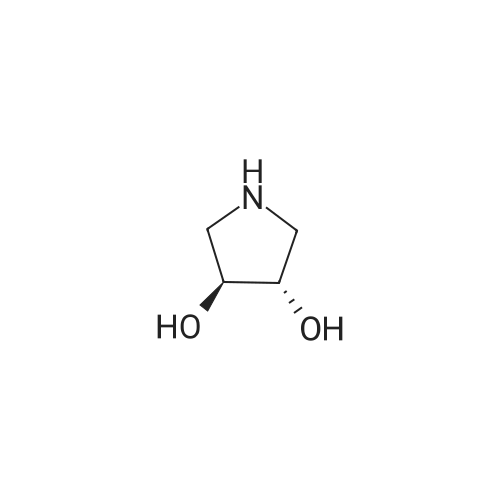
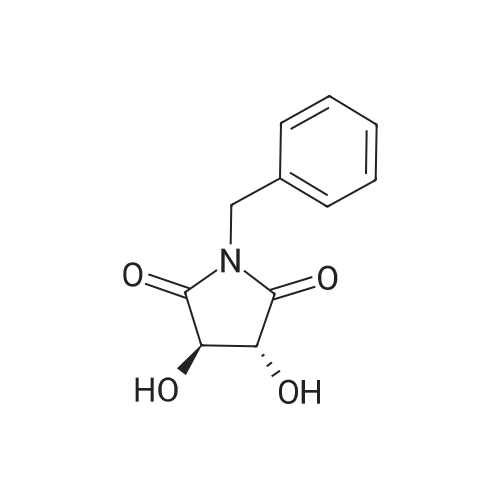
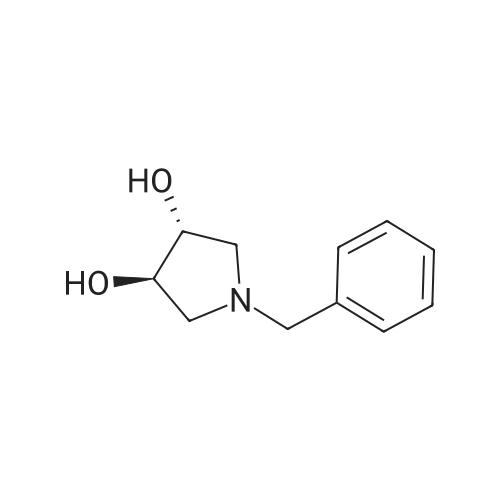
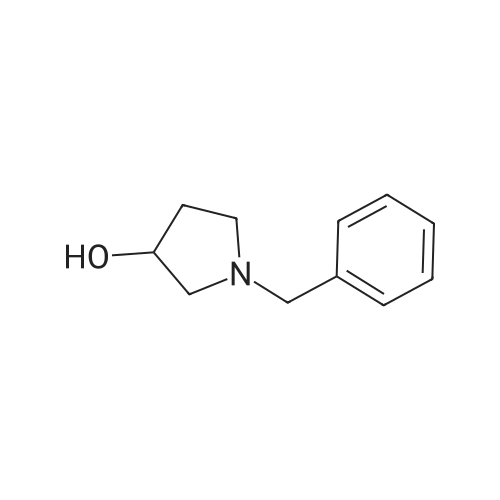
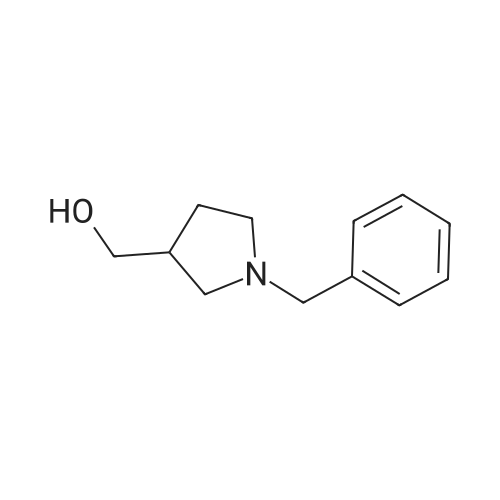
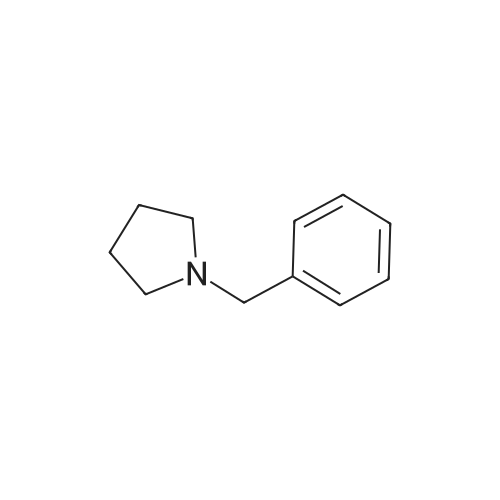
| 71% |
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0℃; for 12h;Inert atmosphere; Reflux; |
Under the protection of nitrogen,The intermediate ((3R, 4R)-4) (132.7 g, 0.6 mol) was slowly added to a solution of LiAlH4 (61.2 g, 1.6 mol) in tetrahydrofuran (3.6 L) after cooling to 0 C.The reaction mixture was refluxed for 12 h.After cooling to room temperature, ethyl acetate (144 mL) was added dropwise in ice water.Distilled water (61.2 mL) was added dropwise under vigorous stirring.5% NaOH (61.2 mL) and distilled water (183.6 mL).Filter and filter cake was washed with hot tetrahydrofuran (2 x 1.2 L).The filtrate and washings were combined and concentrated under reduced pressure.The residue was purified on a silica gel column to give a pale yellow oil.After recrystallization from ethyl acetate, the title compound ((3S, 4S)-5)The yield was 71.0%. |
| 69% |
With lithium aluminium tetrahydride; In tetrahydrofuran; at -5 - 95℃; for 12h; |
Lithium aluminum tetrahydrate (5 g, 132 mmol)Was dissolved in anhydrous tetrahydrofuran (100 ml)A solution of (3R, 4R) -1-benzyl-3,4-dihydroxy-2,5-pyrrolidinedione (10 g, 45 mmol) in dry tetrahydrofuran was added at -5 C. After completion of the dropwise addition, The reaction was allowed to proceed to 95 C for 12 hours,Cooled to room temperature, 6.8 g of water was added dropwise to the reaction system, and the floc was present. Then, 6.8 g of 20% NaoH solution and 20.4 g of water were added. Filtered and purified under reduced pressure and purified by silica gel column chromatography (dichloromethane: methanol = 50: 1)To give 6 g of (3S, 4S) -1-benzyl-3,4-pyrrolidine diol (pale yellow solid in 69% yield). |
| 61% |
|
The (3R,4R)-l-benzyl-3,4-dihydroxypyrrolidine-2,5-dione (0.98 g, 4.4 mmol) <n="124"/>in THF (20 ml) was added slowly to a stirring solution Of LiAlH4 (4.75 ml, 11.87 mmol of 2.5 M solution in THF) in THF cooled to -5C. After complete addition, the reaction was warmed to room temperature, then heated at reflux overnight. The reaction was cooled to room temperature, then quenched with saturated aqueous NH4Cl until further addition produced no more bubbling. The reaction was diluted with ethyl acetate (20 ml), filtered, and the solid washed with ethyl acetate. The combined filtrates were concentrated, and the residue purified by silica flash chromatography (gradient elution, using EtOAc, and 9: 1 EtOAc-EtOH) to provide the title compound as a tan solid (0.52 g, 61%). |
| 45% |
|
To a stirred solution of boron trifluoride ethyl etherate (23 mL, 0.16 mol) in DME (120 mL) were added SLnHK-01-2a (10 g, 0.04 mol) and sodium borohydride (6.2 g, 0.16 mol) at 0 C. The mixture was stirred at 70 C. for 2 h. Then 6 N HCl (62.5 mL) was added slowly at 70 C., stirred for 15 min. Sodium fluoride (28 g) was added and the mixture was heated at reflux temperature for 30 min. The mixture was cooled to room temperature, 20% aq. NaOH (53 mL) was added and the resulting mixture was filtered. The organic phase was isolated, evaporated to dryness and obtained residue was partitioned between water and diethyl ether. The water phase was extracted with diethyl ether (2x100 mL). The combined organic phases were dried over MgSO4, evaporated to dryness and obtained crude material was recrystallized from ethyl acetate to obtain SLnHK-01-3a (S,S) (7.0 g, 45%) as white crystals.1H NMR (200 MHz, CDCl3): delta 7.34-7.25 (m, 5H), 4.04 (t, J=4.2 Hz, 2H), 3.58 (d, J=7.8 Hz, 2H), 2.92-2.88 (m, 2H), 2.44-2.40 (m, 2H). |
| 12% |
With borane-THF; In tetrahydrofuran; at 20 - 60℃; for 2h; |
To a mixture of (3R,4R)-1-benzyl-3,4-dihydroxypyrrolidine-2,5-dione (44 g, 199 mmol, 1.0 eq) and THF (176 mL) at 20 C. (vessel jacket temperature) was added borane-tetrahydrofuran complex (1.0 mol/L) in THF (800 mL, 800 mmol, 1.0 mol/L, 4.0 eq) at a rate to maintain the temperature between 20 C. and 25 C. Over 1 hr, the jacket temperature was ramped to 60 C. and then held for 1 hr. Upon completion, the reaction was cooled to 30 C. and quenched by the slow dropwise addition of MeOH (97 mL, 12 eq) to the mixture at a rate to control off gassing. The reaction mixture was then heated to reflux and concentrated to a low stir volume. The reaction solvent THF was then replaced by a constant volume displacement with MeOH (total of 1.5 L). Once the THF content had been reduced to less than 1 wt %, MeOH was replaced by a constant volume displacement with EtOAc (total of 1.5 L) to reduce the MeOH content to less than 1 wt %. The total volume of EtOAc was then readjusted to about 250 mL (6 vol) and then cooled to 5 C. to crystallize the product. The desired product was isolated by filtration, washed with cold EtOAc (88 mL) and dried to give title compound (27.0 g, 140 mmol, 70%). A second crop of product was isolated by concentration of the combined filtrate and cake wash to half volume, which was then cooled to 5 C., filtered and washed with cold EtOAc (50 mL) to afford additional title compound (4.5 g, 23 mmol, 12%). 1H NMR (400 MHz, DMSO-d6) delta ppm 7.33-7.26 (m, 4H) 7.25-7.20 (m, 1H) 4.48 (d, J=4.8 Hz, 2H) 3.38-3.31 (m, 2H), 3.57 (d, J=13.0 Hz, 1H) 3.46 (d, J=13.0 Hz, 1H) 2.74 (dd, J=9.4, 5.9 Hz, 2H) 2.30 (dd, J=9.4, 4.4 Hz, 2H). m/z (EI+) for C11H15NO2 194.2 (M+H)+ |
|
|
The compound obtained in Referential Example 188 (11 g) was dissolved in tetrahydrofuran (110 mL), and lithium aluminium hydride (5.69 g) was added thereto in small portions under ice cooling. The thus-obtained mixture was heated to room temperature, and after 1 hour, the mixture was heated under reflux overnight. The resultant mixture was left to cool, and to the mixture were sequentially added water (5.7 mL), 15% aqueous sodium hydroxide (5.7 mL), water (17.1 mL) under ice cooling. The reaction mixture was brought back to room temperature, and was stirred for 1 hour. The resultant precipitate was filtered through Celite, and the filtrate was concentrated. The residue was recrystallized from ethyl acetate, to thereby give the title compound (6.35 g).1H-NMR(CDCl3) delta:2.40-2.44(2H, m), 2.88-2.92(2H, m), 3.58(each 1H, AB type d, J=7.8Hz), 4.04(2H, t, J=4.2Hz), 7.25-7.34(5H, m). |
|
With hydrogenchloride; sodium hydroxide; sulfuric acid; In methanol; 1,2-dimethoxyethane; dichloromethane; water; ethyl acetate; toluene; |
Example 6 A solution of 32.2 ml (0.6 mole) sulfuric acid in 100 ml DME was added dropwise to a suspension of 66.4 g (0.3 mole) (3R,4R)-1-benzyl-3,4-dihydroxy-2,5-pyrrolidine dione (IIb) and 45.6 g (1.2 moles) sodium boron hydride in 400 ml 1,2-dimethoxyethane (DME) within 2.5 h at room temperature. The mixture was then agitated 4 h at 75 C. After it cooled off, 75 ml MeOH were added and the mixture rotated in to dryness. The residue was taken up in 300 ml water and compounded with 50 ml conc. hydrochloric acid. The mixture was then made alkaline and extracted with 200 ml toluene twice at 65 C. The extraction residue was taken up in 100 ml each of water and toluene, adjusted to be strongly acidic with hydrochloric acid and agitated overnight at room temperature. It was then made alkaline with 30% sodium hydroxide solution. After the addition of 200 ml toluene the organic phase was separated at 65 C. The aqueous phase was extracted again with 200 ml methylene chloride. After the organic phases were rotated in, 85 g oil remained. The latter was taken up in 400 ml ethyl acetate and washed twice with 80 ml water at 65 C. The ethyl-acetate phase was rotated in after drying over sodium sulfate. 60 g residue remained which was recrystallized from 150 ml ethyl acetate. 30.2 g (52.1%) (3S,4S)-1-benzyl-3,4-dihydroxypyrrolidine (IIIb) were obtained in two fractions. Melting point: 97-98 C. [alpha]D20: +33.5 (1.5, MeOH) C11H15NO2 calc.: C 68.37 H 7.82 N 7.25 193.25 obs.: C 68.30 H 7.98 N 7.32 |
|
With sodium hydroxide; In tetrahydrofuran; water; |
REFERENTIAL EXAMPLE 189 (3S,4S)-1-Benzyl-3,4-pyrrolidinediol: The compound (11 g) obtained in Referential Example 188 was dissolved in tetrahydrofuran (110 ml), and lithium aluminum hydride (5.69 g) was added portionwise to the solution under ice cooling. The mixture was heated to room temperature for 1 hour and heated under reflux and for additional a night. After allowing the reaction mixture to cool, water (5.7 ml), a 15% aqueous solution (5.7 ml) of sodium hydroxide and water (17.1 ml) were added under ice cooling in that order, and the mixture was heated to room temperature and stirred for 1 hour. After deposits were filtered through Celite, and the mother liquor was concentrated under reduced pressure, the resultant residue was recrystallized from ethyl acetate to obtain title compound (6.35 g). 1H-NMR (CDCl3) delta: 2.40-2.44(2H,m), 2.88-2.92(2H,m), 3.58(each 1H,AB type d,J=7.8 Hz), 4.04(2H,t,J=4.2 Hz), 7.25-7.34(5H,m). |
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20℃;Heating / reflux; |
[Referential Example 189] (3S,4S)-1-Benzyl-3,4-pyrrolidinediol: The compound (11 g) obtained in Referential Example 188 was dissolved in tetrahydrofuran (110 ml), and lithium aluminum hydride (5.69 g) was added portionwise to the solution under ice cooling.. The mixture was heated to room temperature for 1 hour and heated under reflux and for additional a night.. After allowing the reaction mixture to cool, water (5.7 ml), a 15% aqueous solution (5.7 ml) of sodium hydroxide and water (17.1 ml) were added under ice cooling in that order, and the mixture was heated to room temperature and stirred for 1 hour.. After deposits were filtered through Celite, and the mother liquor was concentrated under reduced pressure, the resultant residue was recrystallized from ethyl acetate to obtain title compound (6.35 g).1H-NMR (CDCl3) delta: 2.40-2.44(2H,m), 2.88-2.92(2H,m), 3.58(each 1H,AB type d,J=7.8Hz), 4.04(2H,t,J=4.2Hz), 7.25-7.34(5H,m). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping